Identification of integrin beta subunit mutations that alter heterodimer function in situ
- PMID: 15194810
- PMCID: PMC491840
- DOI: 10.1091/mbc.e04-02-0085
Identification of integrin beta subunit mutations that alter heterodimer function in situ
Abstract
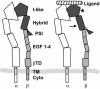
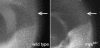
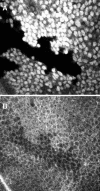
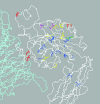
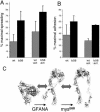
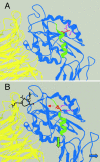
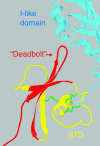
We conducted a genetic screen for mutations in myospheroid, the gene encoding the Drosophila betaPS integrin subunit, and identified point mutants in all of the structural domains of the protein. Surprisingly, we find that mutations in very strongly conserved residues will often allow sufficient integrin function to support the development of adult animals, including mutations in the ADMIDAS site and in a cytoplasmic NPXY motif. Many mutations in the I-like domain reduce integrin expression specifically when betaPS is combined with activating alphaPS2 cytoplasmic mutations, indicating that integrins in the extended conformation are unstable relative to the inactive, bent heterodimers. Interestingly, the screen has identified alleles that show gain-of-function characteristics in cell culture, but have negative effects on animal development or viability. This is illustrated by the allele mys(b58); available structural models suggest that the molecular lesion of mys(b58), V409>D, should promote the "open" conformation of the beta subunit I-like domain. This expectation is supported by the finding that alphaPS2betaPS (V409>D) promotes adhesion and spreading of S2 cells more effectively than does wild-type alphaPS2betaPS, even when betaPS is paired with alphaPS2 containing activating cytoplasmic mutations. Finally, comparisons with the sequence of human beta8 suggest that evolution has targeted the "mys(b58)" residue as a means of affecting integrin activity.
Figures

Similar articles
-
Amino acid changes in Drosophila alphaPS2betaPS integrins that affect ligand affinity.J Biol Chem. 2006 Feb 24;281(8):5050-7. doi: 10.1074/jbc.M508550200. Epub 2005 Dec 21. J Biol Chem. 2006. PMID: 16371365
-
Analysis of the Drosophila betaPS subunit indicates that regulation of integrin activity is a primal function of the C8-C9 loop.Exp Cell Res. 2004 Mar 10;294(1):118-29. doi: 10.1016/j.yexcr.2003.11.002. Exp Cell Res. 2004. PMID: 14980507
-
Disruption of C-terminal cytoplasmic domain of betaPS integrin subunit has dominant negative properties in developing Drosophila.Mol Biol Cell. 2002 Apr;13(4):1352-65. doi: 10.1091/mbc.01-08-0429. Mol Biol Cell. 2002. PMID: 11950944 Free PMC article.
-
Role of the PS integrins in Drosophila development.Immunol Cell Biol. 1995 Dec;73(6):558-64. doi: 10.1038/icb.1995.89. Immunol Cell Biol. 1995. PMID: 8713479 Review.
-
Evolution of integrin I domains.Adv Exp Med Biol. 2014;819:1-19. doi: 10.1007/978-94-017-9153-3_1. Adv Exp Med Biol. 2014. PMID: 25023164 Review.
Cited by
-
The effect of ligand affinity on integrins' lateral diffusion in cultured cells.Eur Biophys J. 2013 Apr;42(4):281-90. doi: 10.1007/s00249-012-0873-x. Epub 2012 Dec 15. Eur Biophys J. 2013. PMID: 23242168
-
Mechanical force regulates integrin turnover in Drosophila in vivo.Nat Cell Biol. 2012 Sep;14(9):935-43. doi: 10.1038/ncb2555. Epub 2012 Aug 12. Nat Cell Biol. 2012. PMID: 22885771
-
Novel tools for genetic manipulation of follicle stem cells in the Drosophila ovary reveal an integrin-dependent transition from quiescence to proliferation.Genetics. 2015 Apr;199(4):935-57. doi: 10.1534/genetics.114.173617. Epub 2015 Feb 12. Genetics. 2015. PMID: 25680813 Free PMC article.
-
The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses.J Cell Biol. 2007 Jun 4;177(5):843-55. doi: 10.1083/jcb.200701111. J Cell Biol. 2007. PMID: 17548512 Free PMC article.
-
Identification of integrin beta subunit mutations that alter affinity for extracellular matrix ligand.J Biol Chem. 2011 Sep 2;286(35):30981-30993. doi: 10.1074/jbc.M111.254797. Epub 2011 Jul 11. J Biol Chem. 2011. PMID: 21757698 Free PMC article.
References
-
- Ashburner, M. (1989). Drosophila: A Laboratory Handbook. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
-
- Beglova, N., Blacklow, S.C., Takagi, J., and Springer, T.A. (2002). Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 9, 282-287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

