Role of the alpha-glucanase Agn1p in fission-yeast cell separation
- PMID: 15194814
- PMCID: PMC491845
- DOI: 10.1091/mbc.e04-04-0319
Role of the alpha-glucanase Agn1p in fission-yeast cell separation
Abstract
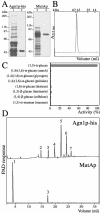
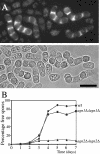
Cell division in the fission yeast Schizosaccharomyces pombe yields two equal-sized daughter cells. Medial fission is achieved by deposition of a primary septum flanked by two secondary septa within the dividing cell. During the final step of cell division, cell separation, the primary septum is hydrolyzed by an endo-(1,3)-beta-glucanase, Eng1p. We reasoned that the cell wall material surrounding the septum, referred to here as the septum edging, also must be hydrolyzed before full separation of the daughter cells can occur. Because the septum edging contains (1,3)-alpha-glucan, we investigated the cellular functions of the putative (1,3)-alpha-glucanases Agn1p and Agn2p. Whereas agn2 deletion results in a defect in endolysis of the ascus wall, deletion of agn1 leads to clumped cells that remained attached to each other by septum-edging material. Purified Agn1p hydrolyzes (1,3)-alpha-glucan predominantly into pentasaccharides, indicating an endo-catalytic mode of hydrolysis. Furthermore, we show that the transcription factors Sep1p and Ace2p regulate both eng1 and agn1 expression in a cell cycle-dependent manner. We propose that Agn1p acts in concert with Eng1p to achieve efficient cell separation, thereby exposing the secondary septa as the new ends of the daughter cells.
Figures






References
-
- Akada, R., Kawahata, M., and Nishizawa, Y. (2000). Elevated temperature greatly improves transformation of fresh and frozen competent cells in yeast. Biotechniques 28, 854-856. - PubMed
-
- Bähler, J., Wu, J.-Q., Longtine, M.S., Shah, N.G., McKenzie, A. 3rd, Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. - PubMed
-
- Cabib, E., Roh, D.-H., Schmidt, M., Crotti, L.B., and Varma, A. (2001). The yeast cell wall and septum as paradigms of cell growth and morphogenesis. J. Biol. Chem. 276, 19679-19682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

