MicroRNA-directed cleavage of Nicotiana sylvestris PHAVOLUTA mRNA regulates the vascular cambium and structure of apical meristems
- PMID: 15194817
- PMCID: PMC514157
- DOI: 10.1105/tpc.021816
MicroRNA-directed cleavage of Nicotiana sylvestris PHAVOLUTA mRNA regulates the vascular cambium and structure of apical meristems
Abstract
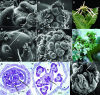
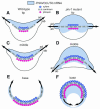
Leaf initiation in the peripheral zone of the shoot apical meristem involves a transition to determinate cell fate, but indeterminacy is maintained in the vascular cambium, a tissue critical to the continuous growth of vascular tissue in leaves and stems. We show that the orientation of cambial growth is regulated by microRNA (miRNA)-directed cleavage of mRNA from the Nicotiana sylvestris ortholog of PHAVOLUTA (NsPHAV). Loss of miRNA regulation in semidominant phv1 mutants misdirects lateral growth of leaf midveins and stem vasculature away from the shoot, disrupting vascular connections in stem nodes. The phv1 mutation also expands the central zone in vegetative and inflorescence meristems, implicating miRNA and NsPHAV in regulation of meristem structure. In flowers, phv1 causes reiteration of carpel initiation, a phenocopy for loss of CARPEL FACTORY/DICER LIKE1, indicating that miRNA is critical to the termination of indeterminacy in floral meristems. Results point to a common role for miRNA in spatial and temporal restriction of HD-ZIPIII mediated indeterminacy in apical and vascular meristems.
Figures



References
-
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585. - PubMed
-
- Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13, 1768–1774. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

