Iron down-regulates macrophage anti-tumour activity by blocking nitric oxide production
- PMID: 15196250
- PMCID: PMC1809097
- DOI: 10.1111/j.1365-2249.2004.02515.x
Iron down-regulates macrophage anti-tumour activity by blocking nitric oxide production
Abstract
Although the inhibitory effect of iron on macrophage production of tumoricidal free radical nitric oxide (NO) has been reported, its possible influence on macrophage anti-tumour activity has not been established. In the present study, FeSO4 markedly reduced IFN-gamma + LPS-induced NO synthesis in mouse and rat macrophages. The effect of iron coincided with the loss of macrophage cytotoxic activity against NO-sensitive C6 rat astrocytoma and L929 mouse fibrosarcoma cell lines, as measured by MTT assay for cellular respiration and the crystal violet test for cell viability. Tumour cell survival did not improve further in the presence of FeSO4 if macrophage NO release and cytotoxicity were already blocked by aminoguanidine. In accordance with the results obtained with exogenous iron, cell membrane permeable iron chelator o-phenanthroline enhanced both macrophage NO release and anti-tumour activity. Iron also down-regulated NO production and increased the viability of L929 fibrosarcoma cells stimulated with IFN-gamma + LPS in the absence of macrophages. However, neither NO release nor cell viability was affected by iron addition to cultures of the C6 astrocytoma cell line. Iron was unable to prevent L929 and C6 cell death induced by the NO releasing chemicals SNP and SIN-1, indicating that iron-mediated inhibition of NO synthesis, rather than interference with its cytotoxic action, was responsible for the protection of tumour cells. Collectively, these results indicate that iron might protect tumour cells by reducing both macrophage and tumour cell-derived NO release.
Figures
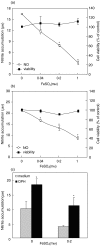
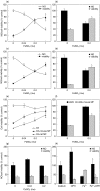
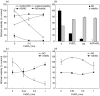
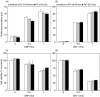
Similar articles
-
Acidosis affects tumor cell survival through modulation of nitric oxide release.Free Radic Biol Med. 2006 Jan 15;40(2):226-35. doi: 10.1016/j.freeradbiomed.2005.08.027. Epub 2005 Nov 18. Free Radic Biol Med. 2006. PMID: 16413405
-
Transforming growth factor-beta 1 primes macrophages for enhanced expression of the nitric oxide synthase gene for nitric oxide-dependent cytotoxicity against Entamoeba histolytica.Immunology. 1995 Jul;85(3):400-7. Immunology. 1995. PMID: 7558128 Free PMC article.
-
IFN-gamma differentially modulates the susceptibility of L1210 and P815 tumor targets for macrophage-mediated cytotoxicity. Role of macrophage-target interaction coupled to nitric oxide generation, but independent of tumor necrosis factor production.J Immunol. 1991 Sep 15;147(6):1816-22. J Immunol. 1991. PMID: 1909732
-
Iron depletion: a defense against intracellular infection and neoplasia.Life Sci. 1992;50(18):1289-97. doi: 10.1016/0024-3205(92)90279-x. Life Sci. 1992. PMID: 1560730 Review.
-
Role of nitric oxide synthesis in macrophage antimicrobial activity.Curr Opin Immunol. 1991 Feb;3(1):65-70. doi: 10.1016/0952-7915(91)90079-g. Curr Opin Immunol. 1991. PMID: 1711326 Review.
Cited by
-
[Effects of liver fibrosis induced by iron overload on M2 polarization of macrophages in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2025 Apr 20;45(4):684-691. doi: 10.12122/j.issn.1673-4254.2025.04.02. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40294917 Free PMC article. Chinese.
-
Redox-Regulated Iron Metabolism and Ferroptosis in Ovarian Cancer: Molecular Insights and Therapeutic Opportunities.Antioxidants (Basel). 2024 Jun 28;13(7):791. doi: 10.3390/antiox13070791. Antioxidants (Basel). 2024. PMID: 39061859 Free PMC article. Review.
-
A closer look at the role of iron in glioblastoma.Neuro Oncol. 2023 Dec 8;25(12):2136-2149. doi: 10.1093/neuonc/noad136. Neuro Oncol. 2023. PMID: 37539622 Free PMC article. Review.
-
Biocorrosion properties and blood and cell compatibility of pure iron as a biodegradable biomaterial.J Mater Sci Mater Med. 2010 Jul;21(7):2151-63. doi: 10.1007/s10856-010-4070-0. Epub 2010 Apr 16. J Mater Sci Mater Med. 2010. PMID: 20396936
-
Risk of cancer in patients with iron deficiency anemia: a nationwide population-based study.PLoS One. 2015 Mar 17;10(3):e0119647. doi: 10.1371/journal.pone.0119647. eCollection 2015. PLoS One. 2015. PMID: 25781632 Free PMC article.
References
-
- Lieu PT, Heiskala M, Peterson PA, Yang Y. The roles of iron in health and disease. Mol Aspects Med. 2001;22:1–87. - PubMed
-
- Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. 1996;20:553–66. - PubMed
-
- Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319:1047–52. - PubMed
-
- Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int J Cancer. 1994;56:364–9. - PubMed
-
- Richardson DR. Iron chelators as therapeutic agents for the treatment of cancer. Crit Rev Oncol Hematol. 2002;42:267–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

