Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats
- PMID: 15197101
- PMCID: PMC1575059
- DOI: 10.1038/sj.bjp.0705811
Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats
Abstract
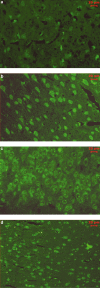
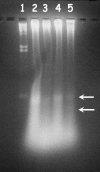
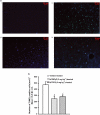
Free radicals have been implicated in cerebral ischemia reperfusion (IR) injury. Massive production of nitric oxide and superoxide results in continuous formation of peroxynitrite even several hours after IR insult. This can produce DNA strand nicks, hydroxylation and/or nitration of cytosolic components of neuron, leading to neuronal death. Peroxynitrite decomposition catalysts 5,10,15,20-tetrakis(N-methyl-4'-pyridyl)porphyrinato iron (III) (FeTMPyP) and 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III) (FeTPPS) have been demonstrated to protect neurons in in vitro cultures; however, their neuroprotective efficacy in cerebral IR injury has not been explored. In the present study, we investigated the efficacy and the therapeutic time window of FeTMPyP and FeTPPS in focal cerebral ischemia (FCI). FCI was induced according to the middle cerebral artery occlusion (MCAO) method. After 2 h of MCAO and 70 h of reperfusion, the extent of neurological deficits, infarct and edema volume were measured in Sprague-Dawley rats. FeTMPyP and FeTPPS were administered at different time points 2, 6, 9 and 12 h post MCAO. FeTMPyP and FeTPPS (3 mg kg(-1), i.v.) treatment at 2 and 6 h post MCAO produced significant reduction in infarct volume, edema volume and neurological deficits. However, treatment at latter time points did not produce significant neuroprotection. Significant reduction of peroxynitrite in blood and nitrotyrosine in brain sections was observed on FeTMPyP and FeTPPS treatment. As delayed treatment of FeTMPyP and FeTPPS produced neuroprotection, we tested whether treatment had any influence over the apoptotic neuronal death. DNA fragmentation and in situ nick end-labeling assays showed that FeTMPyP and FeTPPS treatment reduced IR injury-induced DNA fragmentation. In conclusion, peroxynitrite decomposition catalysts (FeTMPyP and FeTPPS) produced prominent neuroprotection even if administered 6 h post MCAO and the neuroprotective effect is at least in part due to the reduction of peroxynitrite and apoptosis.
Figures

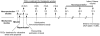



Similar articles
-
Neuroprotective effects of NU1025, a PARP inhibitor in cerebral ischemia are mediated through reduction in NAD depletion and DNA fragmentation.Life Sci. 2006 Nov 10;79(24):2293-302. doi: 10.1016/j.lfs.2006.07.034. Epub 2006 Aug 2. Life Sci. 2006. PMID: 16935310
-
Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of Sophora flavescens Ait. against focal cerebral ischemia in rats.Phytomedicine. 2009 Nov;16(11):1042-51. doi: 10.1016/j.phymed.2009.03.017. Epub 2009 May 8. Phytomedicine. 2009. PMID: 19427179
-
Neuroprotective effect of 4-amino-1,8-napthalimide, a poly(ADP ribose) polymerase inhibitor in middle cerebral artery occlusion-induced focal cerebral ischemia in rat.Brain Res Bull. 2004 Feb 1;62(5):425-33. doi: 10.1016/j.brainresbull.2003.11.001. Brain Res Bull. 2004. PMID: 15168908
-
Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts.Ann N Y Acad Sci. 2001 Jun;939:366-80. doi: 10.1111/j.1749-6632.2001.tb03646.x. Ann N Y Acad Sci. 2001. PMID: 11462792 Review.
-
Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia.Cell Biochem Biophys. 2003;39(1):13-22. doi: 10.1385/CBB:39:1:13. Cell Biochem Biophys. 2003. PMID: 12835526 Review.
Cited by
-
S-nitrosoglutathione reduces oxidative injury and promotes mechanisms of neurorepair following traumatic brain injury in rats.J Neuroinflammation. 2011 Jul 6;8:78. doi: 10.1186/1742-2094-8-78. J Neuroinflammation. 2011. PMID: 21733162 Free PMC article.
-
Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity.Free Radic Biol Med. 2006 Jun 1;40(11):1960-70. doi: 10.1016/j.freeradbiomed.2006.01.022. Epub 2006 Feb 17. Free Radic Biol Med. 2006. PMID: 16716897 Free PMC article.
-
INO-4885 [5,10,15,20-tetra[N-(benzyl-4'-carboxylate)-2-pyridinium]-21H,23H-porphine iron(III) chloride], a peroxynitrite decomposition catalyst, protects the heart against reperfusion injury in mice.J Pharmacol Exp Ther. 2009 Mar;328(3):777-84. doi: 10.1124/jpet.108.144352. Epub 2008 Nov 25. J Pharmacol Exp Ther. 2009. PMID: 19033557 Free PMC article.
-
Peroxynitrite decomposition with FeTMPyP improves plasma-induced vascular dysfunction and infarction during mild but not severe hyperglycemic stroke.J Cereb Blood Flow Metab. 2012 Jun;32(6):1035-45. doi: 10.1038/jcbfm.2012.14. Epub 2012 Feb 29. J Cereb Blood Flow Metab. 2012. PMID: 22373645 Free PMC article.
-
Design, mechanism of action, bioavailability and therapeutic effects of mn porphyrin-based redox modulators.Med Princ Pract. 2013;22(2):103-30. doi: 10.1159/000341715. Epub 2012 Oct 16. Med Princ Pract. 2013. PMID: 23075911 Free PMC article. Review.
References
-
- AKIYAMA K., SUZUKI H., GRANT P., BING R.J. Oxidation products of nitric oxide, NO2 and NO3, in plasma after experimental myocardial infarction. J. Mol. Cell. Cardiol. 1997;29:1–9. - PubMed
-
- BECKMAN J.S., CHEN J., CROW J.P., YE Y.Z. Reactions of nitric oxide, superoxide and peroxynitrite with superoxide dismutase in neurodegeneration. Prog. Brain Res. 1994;103:371–380. - PubMed
-
- BECKMAN J.S., ISCHIROPOULOS H., ZHU L., VAN DER WOERD M., SMITH C., CHEN J., HARRISON J., MARTIN J.C., TSAI M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys. 1992;298:438–445. - PubMed
-
- BIANCHI C., WAKIYAMA H., FARO R., KHAN T., MCCULLY J.D., LEVITSKY S., SZABO C., SELLKE F.W. A novel peroxynitrite decomposer catalyst (FP-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia–reperfusion in pigs. Ann. Thorac. Surg. 2002;74:1201–1207. - PubMed
-
- CASTRO L., RODRIGUEZ M., RADI R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J. Biol. Chem. 1994;269:29409–29415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

