Phosphorylation of histone H2B at DNA double-strand breaks
- PMID: 15197225
- PMCID: PMC2212807
- DOI: 10.1084/jem.20032247
Phosphorylation of histone H2B at DNA double-strand breaks
Abstract
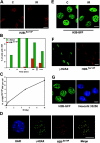
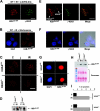
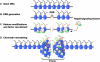
Posttranslational modifications of histone tails regulate numerous biological processes including transcription, DNA repair, and apoptosis. Although recent studies suggest that structural alterations in chromatin are critical for triggering the DNA damage response, very little is known about the nature of DNA damage-induced chromatin perturbations. Here we show that the serine 14 residue in the NH(2)-terminal tail of histone H2B is rapidly phosphorylated at sites of DNA double-strand breaks. At late time points after irradiation, the phosphorylated form of H2B, H2B-(Ser14P), accumulates into irradiation-induced foci. H2B-(Ser14P) foci formation is not associated with the apoptotic phosphorylation of H2B but is strictly dependent on the phosphorylated isoform of H2AX. Our results broaden the spectrum of histone modifications that constitute the DNA damage "histone code" and suggest a model for the underlying chromatin structure within damage-induced foci.
Figures
References
-
- Bakkenist, C.J., and M.B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 421:499–506. - PubMed
-
- Jenuwein, T., and C.D. Allis. 2001. Translating the histone code. Science. 293:1074–1080. - PubMed
-
- Rogakou, E.P., D.R. Pilch, A.H. Orr, V.S. Ivanova, and W.M. Bonner. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273:5858–5868. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

