Direct imaging shows that insulin granule exocytosis occurs by complete vesicle fusion
- PMID: 15197259
- PMCID: PMC438965
- DOI: 10.1073/pnas.0403201101
Direct imaging shows that insulin granule exocytosis occurs by complete vesicle fusion
Abstract
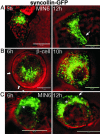
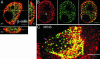
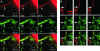
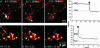
Confocal imaging of GFP-tagged secretory granules combined with the use of impermeant extracellular dyes permits direct observation of insulin packaged in secretory granules, trafficking of these granules to the plasma membrane, exocytotic fusion of granules with the plasma membrane, and eventually the retrieval of membranes by endocytosis. Most such studies have been done in tumor cell lines, using either confocal methods or total internal reflectance microscopy. Here we compared these methods by using GFP-syncollin or PC3-GFP plus rhodamine dextrans to study insulin granule dynamics in insulinoma cells, normal mouse islets, and primary pancreatic beta cells. We found that most apparently docked granules did not fuse with the plasma membrane after stimulation. Granules that did fuse typically fused completely, but a few dextran-filled granules lingered at the membrane. Direct recycling of granules occurred only rarely. Similar results were obtained with both confocal and total internal reflection microscopy, although each technique had advantages for particular aspects of the granule life cycle. We conclude that insulin exocytosis involves a prolonged interaction of secretory granules with the plasma membrane, and that the majority of exocytotic events occur by full, not partial, fusion.
Figures


Similar articles
-
Site of docking and fusion of insulin secretory granules in live MIN6 beta cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy.J Biol Chem. 2004 Feb 27;279(9):8403-8. doi: 10.1074/jbc.M308954200. Epub 2003 Dec 15. J Biol Chem. 2004. PMID: 14676208
-
TIRF imaging of docking and fusion of single insulin granule motion in primary rat pancreatic beta-cells: different behaviour of granule motion between normal and Goto-Kakizaki diabetic rat beta-cells.Biochem J. 2004 Jul 1;381(Pt 1):13-8. doi: 10.1042/BJ20040434. Biochem J. 2004. PMID: 15128287 Free PMC article.
-
The insulin secretory granule is the major site of K(ATP) channels of the endocrine pancreas.Diabetes. 2003 Mar;52(3):767-76. doi: 10.2337/diabetes.52.3.767. Diabetes. 2003. PMID: 12606519
-
Heterogeneous modes of insulin granule exocytosis: molecular determinants.Front Biosci (Landmark Ed). 2011 Jan 1;16(1):360-7. doi: 10.2741/3692. Front Biosci (Landmark Ed). 2011. PMID: 21196175 Review.
-
Insulin secretion by 'kiss-and-run' exocytosis in clonal pancreatic islet beta-cells.Biochem Soc Trans. 2003 Aug;31(Pt 4):833-6. doi: 10.1042/bst0310833. Biochem Soc Trans. 2003. PMID: 12887316 Review.
Cited by
-
Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells.J Cell Sci. 2005 Oct 15;118(Pt 20):4797-812. doi: 10.1242/jcs.02573. J Cell Sci. 2005. PMID: 16219687 Free PMC article.
-
Dominant-negative PKC-epsilon impairs apical actin remodeling in parallel with inhibition of carbachol-stimulated secretion in rabbit lacrimal acini.Am J Physiol Cell Physiol. 2005 Oct;289(4):C1052-68. doi: 10.1152/ajpcell.00546.2004. Epub 2005 Jun 1. Am J Physiol Cell Physiol. 2005. PMID: 15930141 Free PMC article.
-
Dynamics of insulin secretion and the clinical implications for obesity and diabetes.J Clin Invest. 2011 Jun;121(6):2118-25. doi: 10.1172/JCI45680. Epub 2011 Jun 1. J Clin Invest. 2011. PMID: 21633180 Free PMC article. Review.
-
The changing view of insulin granule mobility: From conveyor belt to signaling hub.Front Endocrinol (Lausanne). 2022 Sep 2;13:983152. doi: 10.3389/fendo.2022.983152. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36120467 Free PMC article. Review.
-
Insulin secretion from beta cells within intact islets: location matters.Clin Exp Pharmacol Physiol. 2015 Apr;42(4):406-14. doi: 10.1111/1440-1681.12368. Clin Exp Pharmacol Physiol. 2015. PMID: 25676261 Free PMC article. Review.
References
-
- Kasai, H. (1999) Trends Neurosci. 22, 88-93. - PubMed
-
- Barg, S. Eliasson, L., Renstrom, E. & Rorsman, P. (2002) Diabetes 51, S74-82. - PubMed
-
- Bratanova-Tochkova, T. K., Cheng, H. Daniel, S., Gunawardana, S., Liu, Y. J., Mulvaney-Musa, J., Schermerhorn, T., Straub, S. G., Yajima, H. & Sharp, G. W. (2002) Diabetes 51, S83-S90. - PubMed
-
- Dean, P. M. (1993) Diabetologia 9, 115-119. - PubMed
-
- Lang, J. (1999) Eur. J. Biochem. 259, 3-17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

