Immunodominant CD4+ responses identified in a patient vaccinated with full-length NY-ESO-1 formulated with ISCOMATRIX adjuvant
- PMID: 15197261
- PMCID: PMC438982
- DOI: 10.1073/pnas.0403271101
Immunodominant CD4+ responses identified in a patient vaccinated with full-length NY-ESO-1 formulated with ISCOMATRIX adjuvant
Abstract
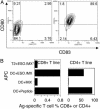
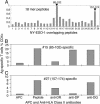
There is increasing evidence showing the involvement of CD4(+) T cells in initiating and maintaining antitumor immune responses. NY-ESO-1 is expressed by various tumors but not normal tissues except testis. We conducted a cancer clinical trial by using full-length NY-ESO-1 protein formulated with ISCOMATRIX adjuvant and injected into patients intramuscularly. Autologous dendritic cells pulsed with NY-ESO-1 ISCOMATRIX in combination with overlapping synthetic peptides were used to identify immunodominant T cells from a vaccinated patient. We show here the identification and characterization of two novel CD4(+) T cell epitopes. T cells specific to these epitopes not only recognized autologous dendritic cells loaded with NY-ESO-1 but also NY-ESO-1-expressing tumor cell lines treated with IFN-gamma. One of the two responses identified was greater than the previously identified immunodominant HLA-DP4-restricted response and correlated with NY-ESO-1-specific CD8(+) T cell induction after vaccination. This T cell response was vaccinated in most patients who expressed HLA-DR2. This study has systematically surveyed patients vaccinated with full-length tumor antigen for a vaccinated CD4 helper T cell response.
Figures



References
-
- Bennett, S. R., Carbone, F. R., Karamalis, F., Flavell, R. A., Miller, J. F. & Heath, W. R. (1998) Nature 393, 478-480. - PubMed
-
- Fearon, E. R., Pardoll, D. M., Itaya, T., Golumbek, P., Levitsky, H. I., Simons, J. W., Karasuyama, H., Vogelstein, B. & Frost, P. (1990) Cell 60, 397-403. - PubMed
-
- Gao, F. G., Khammanivong, V., Liu, W. J., Leggatt, G. R., Frazer, I. H. & Fernando, G. J. (2002) Cancer Res. 62, 6438-6441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

