Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries
- PMID: 15197275
- PMCID: PMC438952
- DOI: 10.1073/pnas.0400187101
Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries
Abstract
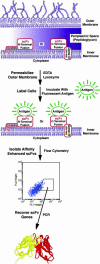
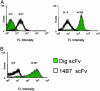
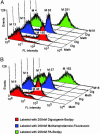
Anchored periplasmic expression (APEx) is a technology for the isolation of ligand-binding proteins from combinatorial libraries anchored on the periplasmic face of the inner membrane of Escherichia coli. After disruption of the outer membrane by Tris-EDTA-lysozyme, the inner-membrane-anchored proteins readily bind fluorescently labeled ligands as large as 240 kDa. Fluorescently labeled cells are isolated by flow cytometry, and the DNA of isolated clones is rescued by PCR. By using two rounds of APEx, the affinity of a neutralizing antibody to the Bacillus anthracis protective antigen was improved >200-fold, exhibiting a final K(D) of 21 pM. This approach has several technical advantages compared with previous library screening technologies, including the unique ability to screen for ligand-binding proteins that bind endogenously expressed ligands fused to a short-lived GFP. Further, APEx is able to display proteins either as an N-terminal fusion to a six-residue sequence derived from the native E. coli lipoprotein NlpA, or as a C-terminal fusion to the phage gene three minor coat protein of M13. The latter fusions allow hybrid phage display/APEx strategies without the need for further subcloning.
Figures


Similar articles
-
Binding and enrichment of Escherichia coli spheroplasts expressing inner membrane tethered scFv antibodies on surface immobilized antigens.Biotechnol Bioeng. 2007 Sep 1;98(1):39-47. doi: 10.1002/bit.21405. Biotechnol Bioeng. 2007. PMID: 17657772
-
Isolation of high-affinity ligand-binding proteins by periplasmic expression with cytometric screening (PECS).Nat Biotechnol. 2001 Jun;19(6):537-42. doi: 10.1038/89281. Nat Biotechnol. 2001. PMID: 11385457
-
Engineering of recombinant antibody fragments to methamphetamine by anchored periplasmic expression.J Immunol Methods. 2006 Jan 20;308(1-2):43-52. doi: 10.1016/j.jim.2005.09.017. Epub 2005 Nov 22. J Immunol Methods. 2006. PMID: 16337958
-
Enrichment of Escherichia coli spheroplasts displaying scFv antibodies specific for antigens expressed on the human cell surface.Appl Microbiol Biotechnol. 2010 Dec;88(6):1385-91. doi: 10.1007/s00253-010-2861-3. Epub 2010 Sep 28. Appl Microbiol Biotechnol. 2010. PMID: 20878322
-
Isolation of engineered, full-length antibodies from libraries expressed in Escherichia coli.Nat Biotechnol. 2007 May;25(5):563-5. doi: 10.1038/nbt1296. Epub 2007 Apr 15. Nat Biotechnol. 2007. PMID: 17435747
Cited by
-
Discovery of aminoacyl-tRNA synthetase activity through cell-surface display of noncanonical amino acids.Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10180-10185. doi: 10.1073/pnas.0601167103. Epub 2006 Jun 26. Proc Natl Acad Sci U S A. 2006. PMID: 16801548 Free PMC article.
-
An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence.Nat Commun. 2019 Nov 6;10(1):5031. doi: 10.1038/s41467-019-13108-2. Nat Commun. 2019. PMID: 31695028 Free PMC article.
-
BrkAutoDisplay: functional display of multiple exogenous proteins on the surface of Escherichia coli by using BrkA autotransporter.Microb Cell Fact. 2015 Sep 4;14:129. doi: 10.1186/s12934-015-0316-3. Microb Cell Fact. 2015. PMID: 26337099 Free PMC article.
-
Anti-human fibroblast growth factor-21 monoclonal antibody preparation, characterization and analysis of in vitro bioactivity.Exp Ther Med. 2016 Nov;12(5):3417-3424. doi: 10.3892/etm.2016.3753. Epub 2016 Sep 27. Exp Ther Med. 2016. PMID: 27882173 Free PMC article.
-
Progress towards recombinant anti-infective antibodies.Recent Pat Antiinfect Drug Discov. 2009 Jan;4(1):1-17. doi: 10.2174/157489109787236319. Recent Pat Antiinfect Drug Discov. 2009. PMID: 19149692 Free PMC article. Review.
References
-
- Hudson, P. J. & Souriau, C. (2003) Nat. Med. 9, 129-134. - PubMed
-
- Brekke, O. H. & Loset, G. A. (2003) Curr. Opin. Pharmacol. 3, 544-550. - PubMed
-
- Maynard, J. A., Maassen, C. B., Leppla, S. H., Brasky, K., Patterson, J. L., Iverson, B. L. & Georgiou, G. (2002) Nat. Biotechnol. 20, 597-601. - PubMed
-
- Hoess, R. H. (2001) Chem. Rev. 101, 3205-3218. - PubMed
-
- Hayhurst, A. & Georgiou, G. (2001) Curr. Opin. Chem. Biol. 5, 683-689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

