Rapid response research to emerging infectious diseases: lessons from SARS
- PMID: 15197395
- PMCID: PMC7097457
- DOI: 10.1038/nrmicro930
Rapid response research to emerging infectious diseases: lessons from SARS
Abstract
New and emerging infectious diseases continue to plague the world, and there is significant concern that recombinant infectious agents can be used as bioterrorism threats. Microbiologists are increasingly being asked to apply their scientific knowledge to respond to these threats. The recent pandemic caused by the severe acute respiratory syndrome (SARS) coronavirus illustrated not only how a newly evolved pathogen can rapidly spread throughout the world but also how the global community can unite to identify the causative agent and control its spread. Rapid response research mechanisms, such as those used by the SARS Accelerated Vaccine Initiative (SAVI), have shown that the application of emergency management techniques, together with rapid response research, can be highly effective when applied appropriately to new infectious diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
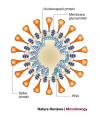
References
-
- World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). [online], <http://www.who.int/csr/sars/en/WHOconsensus.pdf> (2003).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

