Heparan sulphate proteoglycans interact with neurocan and promote neurite outgrowth from cerebellar granule cells
- PMID: 15198637
- PMCID: PMC1134051
- DOI: 10.1042/BJ20040585
Heparan sulphate proteoglycans interact with neurocan and promote neurite outgrowth from cerebellar granule cells
Abstract
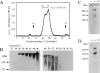
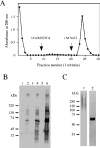
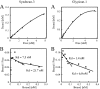
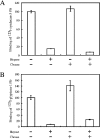
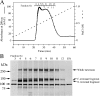
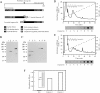
We found that neurocan, a major brain chondroitin sulphate proteoglycan, interacts with HSPGs (heparan sulphate proteoglycans) such as syndecan-3 and glypican-1. Binding of these HSPGs to neurocan was prevented by treatment of the HSPGs with heparitinases I and II, but not by treatment of neurocan with chondroitinase ABC. Scatchard plot analysis indicated that neurocan has two binding sites for these HSPGs with different affinities. It is known that neurocan in the rodent brain is proteolytically processed with aging into N- and C-terminal fragments. When a mixture of whole neurocan and N- and C-terminal fragments prepared from neonatal mouse brains or recombinant N- and C-terminal fragments was applied to a heparin column, the whole molecule and both the N- and C-terminal fragments bound to heparin. A centrifugation cell adhesion assay indicated that both the N- and C-terminal neurocan fragments could interact with these HSPGs expressed on the cell surface. To examine the biological significance of the HSPG-neurocan interaction, cerebellar granule cells expressing these HSPGs were cultured on the recombinant neurocan substrate. A significant increase in the rate of neurite outgrowth was observed on the wells coated with the C-terminal neurocan fragment, but not with the N-terminal one. Neurite outgrowth-promoting activity was inhibited by pretreatment of neurocan substrate with heparin or the addition of heparitinase I to culture medium. These results suggest that HSPGs such as syndecan-3 and glypican-1 serve as the cell-surface receptor of neurocan, and that the interaction of these HSPGs with neurocan through its C-terminal domain is involved in the promotion of neurite outgrowth.
Figures

References
-
- Carey D. J. N-syndecan: structure and function of a transmembrane heparan sulfate proteoglycan. Perspect. Dev. Neurobiol. 1996;3:331–346. - PubMed
-
- Chernousov M. A., Stahl R. C., Carey D. J. Schwann cells secrete a novel collagen-like adhesive protein that binds N-syndecan. J. Biol. Chem. 1996;271:13844–13853. - PubMed
-
- Kinnunen T., Kaksonen M., Saarinen J., Kalkkinen N., Peng H. B., Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 1998;273:10702–10708. - PubMed
-
- Erdman R., Stahl R. C., Rothblum K., Chernousov M. A., Carey D. J. Schwann cell adhesion to a novel heparan sulfate binding site in the N-terminal domain of alpha 4 type V collagen is mediated by syndecan-3. J. Biol. Chem. 2002;277:7619–7625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

