Mechanisms for the acquisition of habitual bipedality: are there biomechanical reasons for the acquisition of upright bipedal posture?
- PMID: 15198701
- PMCID: PMC1571303
- DOI: 10.1111/j.0021-8782.2004.00303.x
Mechanisms for the acquisition of habitual bipedality: are there biomechanical reasons for the acquisition of upright bipedal posture?
Abstract
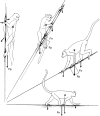
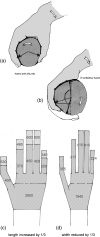
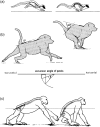
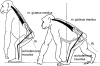
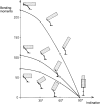
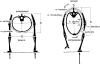
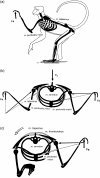
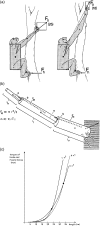
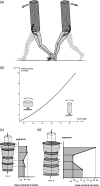
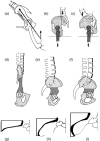
Morphology and biomechanics are linked by causal morphogenesis ('Wolff's law') and the interplay of mutations and selection (Darwin's 'survival of the fittest'). Thus shape-based selective pressures can be determined. In both cases we need to know which biomechanical factors lead to skeletal adaptation, and which ones exert selective pressures on body shape. Each bone must be able to sustain the greatest regularly occurring loads. Smaller loads are unlikely to lead to adaptation of morphology. The highest loads occur primarily in posture and locomotion, simply because of the effect of body weight (or its multiple). In the skull, however, it is biting and chewing that result in the greatest loads. Body shape adapted for an arboreal lifestyle also smooths the way towards bipedality. Hindlimb dominance, length of the limbs in relation to the axial skeleton, grasping hands and feet, mass distribution (especially of the limb segments), thoracic shape, rib curvatures, and the position of the centre of gravity are the adaptations to arboreality that also pre-adapt for bipedality. Five divergent locomotor/morphological types have evolved from this base: arm-swinging in gibbons, forelimb-dominated slow climbing in orangutans, quadrupedalism/climbing in the African apes, an unknown mix of climbing and bipedal walking in australopithecines, and the remarkably endurant bipedal walking of humans. All other apes are also facultative bipeds, but it is the biomechanical characteristics of bipedalism in orangutans, the most arboreal great ape, which is closest to that in humans. If not evolutionary accident, what selective factor can explain why two forms adopted bipedality? Most authors tend to connect bipedal locomotion with some aspect of progressively increasing distance between trees because of climatic changes. More precise factors, in accordance with biomechanical requirements, include stone-throwing, thermoregulation or wading in shallow water. Once bipedality has been acquired, development of typical human morphology can readily be explained as adaptations for energy saving over long distances. A paper in this volume shows that load-carrying ability was enhanced from australopithecines to Homo ergaster (early African H. erectus), supporting an earlier proposition that load-carrying was an essential factor in human evolution.
Figures

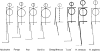

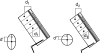


Similar articles
-
Locomotion and posture from the common hominoid ancestor to fully modern hominins, with special reference to the last common panin/hominin ancestor.J Anat. 2008 Apr;212(4):501-43. doi: 10.1111/j.1469-7580.2008.00870.x. J Anat. 2008. PMID: 18380868 Free PMC article. Review.
-
Locomotion in bonobos (Pan paniscus): differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes.J Anat. 2004 May;204(5):353-61. doi: 10.1111/j.0021-8782.2004.00292.x. J Anat. 2004. PMID: 15198700 Free PMC article. Review.
-
The role of load-carrying in the evolution of modern body proportions.J Anat. 2004 May;204(5):417-30. doi: 10.1111/j.0021-8782.2004.00295.x. J Anat. 2004. PMID: 15198704 Free PMC article.
-
Biomechanics and the origins of human bipedal walking: The last 50 years.J Biomech. 2023 Aug;157:111701. doi: 10.1016/j.jbiomech.2023.111701. Epub 2023 Jul 4. J Biomech. 2023. PMID: 37451208 Review.
-
Hip extensor mechanics and the evolution of walking and climbing capabilities in humans, apes, and fossil hominins.Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):4134-4139. doi: 10.1073/pnas.1715120115. Epub 2018 Apr 2. Proc Natl Acad Sci U S A. 2018. PMID: 29610309 Free PMC article.
Cited by
-
Segmental morphometrics of bonobos (Pan paniscus): are they really different from chimpanzees (Pan troglodytes)?J Anat. 2018 Dec;233(6):843-853. doi: 10.1111/joa.12894. Epub 2018 Oct 7. J Anat. 2018. PMID: 30294787 Free PMC article.
-
Delegation to automaticity: the driving force for cognitive evolution?Front Neurosci. 2014 Apr 29;8:90. doi: 10.3389/fnins.2014.00090. eCollection 2014. Front Neurosci. 2014. PMID: 24808820 Free PMC article.
-
A new look at the Dynamic Similarity Hypothesis: the importance of swing phase.Biol Open. 2013 Aug 19;2(10):1032-6. doi: 10.1242/bio.20135165. eCollection 2013. Biol Open. 2013. PMID: 24167713 Free PMC article.
-
Anatomical Pelvic Parameters Using the Anterior Pelvic Plane: Relationships with Standing Sagittal Spinal Alignment and Estimated Lumbar Alignment in Healthy Volunteers.Spine Surg Relat Res. 2024 Dec 20;9(4):469-476. doi: 10.22603/ssrr.2024-0283. eCollection 2025 Jul 27. Spine Surg Relat Res. 2024. PMID: 40786923 Free PMC article.
-
Locomotive Syndrome: Definition and Management.Clin Rev Bone Miner Metab. 2016;14(2):56-67. doi: 10.1007/s12018-016-9208-2. Epub 2016 May 25. Clin Rev Bone Miner Metab. 2016. PMID: 27375370 Free PMC article. Review.
References
-
- Alexander R McN%. Walking and running. Am. Sci. 1984a;72:348–354.
-
- Alexander R McN%. Human walking and running. J. Biol. Education. 1984b;18:135–140.
-
- Amtmann E. Biomechanical interpretation of form and structure of bones: role of genetics and function in growth and remodeling. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behaviour and Morphology: Dynamic Interactions in Primates. New York:: G Fischer.; 1979. pp. 347–366.
-
- Arms A, Voges D, Fischer MS, Preuschoft H. Arboreal locomotion in small New-World monkeys. Z. Morph. Anthropol. 2002;83:243–263. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

