Segmental identity and cerebellar granule cell induction in rhombomere 1
- PMID: 15198802
- PMCID: PMC446226
- DOI: 10.1186/1741-7007-2-14
Segmental identity and cerebellar granule cell induction in rhombomere 1
Abstract
Background: Cerebellar granule cell precursors are specifically generated within the hindbrain segment, rhombomere 1, which is bounded rostrally by the midbrain/hindbrain isthmus and caudally by the boundary of the Hoxa2 expression domain. While graded signals from the isthmus have a demonstrable patterning role within this region, the significance of segmental identity for neuronal specification within rhombomere 1 is unexplored. We examined the response of granule cell precursors to the overexpression of Hoxa2, which normally determines patterns of development specific to the hindbrain. How much does the development of the cerebellum, a midbrain/hindbrain structure, reflect its neuromeric origin as a hindbrain segment?
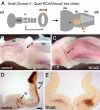
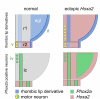
Results: We show that a Gbx2-positive, Otx2-/Hoxa2-negative territory corresponding to rhombomere 1 forms prior to an identifiable isthmic organiser. Early global overexpression of Hoxa2 at embryonic day 0 has no effect on the expression of isthmic signalling molecules or the allocation of rhombomere 1 territory, but selectively results in the loss of granule cell markers at embryonic day 6 and the depletion of cell bodies from the external granule cell layer. By comparison the trochlear nucleus and locus coeruleus form normally in ventral rhombomere 1 under these conditions. Microsurgery, coupled with electroporation, to target Hoxa2 overexpression to rhombic lip precursors, reveals a profound, autonomous respecification of migration. Rhombic lip derivatives, normally destined to occupy the external granule cell layer, violate the cerebellar boundary to form a ventrolateral nucleus in a position comparable to that occupied by rhombic lip derived neurons in rhombomere 2.
Conclusions: Different overexpression strategies reveal that the recognition of migration cues by granule cell precursors is dependent on their identity as rhombomere 1 derivatives. Segmental patterning cues operate autonomously within the rhombic lip precursor pool. By contrast, a subset of coextensive nuclei is refractory to ectopic Hoxa2 and is presumably induced solely by isthmic organiser activity. Thus, graded (isthmic) and segmental mechanisms may operate exclusively of one another in the specification of different neuronal populations within rhombomere 1. The early designation of an Otx2-negative, Hoxa2-negative region, prior to the appearance of the isthmic organiser, is a key initial step in the specification of the cerebellum.
Figures





Similar articles
-
Independently specified Atoh1 domains define novel developmental compartments in rhombomere 1.Development. 2014 Jan;141(2):389-98. doi: 10.1242/dev.099119. Development. 2014. PMID: 24381197 Free PMC article.
-
The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain.J Neurosci. 2001 Jan 1;21(1):159-68. doi: 10.1523/JNEUROSCI.21-01-00159.2001. J Neurosci. 2001. PMID: 11150332 Free PMC article.
-
Fgf8 and Gbx2 induction concomitant with Otx2 repression is correlated with midbrain-hindbrain fate of caudal prosencephalon.Development. 1999 Jun;126(14):3191-203. doi: 10.1242/dev.126.14.3191. Development. 1999. PMID: 10375509
-
Specification of the meso-isthmo-cerebellar region: the Otx2/Gbx2 boundary.Brain Res Brain Res Rev. 2005 Sep;49(2):134-49. doi: 10.1016/j.brainresrev.2005.01.010. Epub 2005 Mar 16. Brain Res Brain Res Rev. 2005. PMID: 16111544 Review.
-
[Formation of the boundary between the midbrain and the hindbrain: involvement of Otx2 and Gbx2 genes].J Soc Biol. 2000;194(3-4):113-8. J Soc Biol. 2000. PMID: 11324311 Review. French.
Cited by
-
Rhombomere-specific analysis reveals the repertoire of genetic cues expressed across the developing hindbrain.Neural Dev. 2009 Feb 10;4:6. doi: 10.1186/1749-8104-4-6. Neural Dev. 2009. PMID: 19208226 Free PMC article.
-
Independently specified Atoh1 domains define novel developmental compartments in rhombomere 1.Development. 2014 Jan;141(2):389-98. doi: 10.1242/dev.099119. Development. 2014. PMID: 24381197 Free PMC article.
-
What cerebellar malformations tell us about cerebellar development.Neurosci Lett. 2019 Jan 1;688:14-25. doi: 10.1016/j.neulet.2018.05.032. Epub 2018 May 23. Neurosci Lett. 2019. PMID: 29802918 Free PMC article. Review.
-
Congenital hypoplasia of the cerebellum: developmental causes and behavioral consequences.Front Neuroanat. 2013 Sep 3;7:29. doi: 10.3389/fnana.2013.00029. Front Neuroanat. 2013. PMID: 24027500 Free PMC article. Review.
-
Embryology.Handb Clin Neurol. 2018;154:29-44. doi: 10.1016/B978-0-444-63956-1.00002-3. Handb Clin Neurol. 2018. PMID: 29903446 Free PMC article. Review.
References
-
- Wingate RJT, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

