Hoxb1 functions in both motoneurons and in tissues of the periphery to establish and maintain the proper neuronal circuitry
- PMID: 15198977
- PMCID: PMC443517
- DOI: 10.1101/gad.1207204
Hoxb1 functions in both motoneurons and in tissues of the periphery to establish and maintain the proper neuronal circuitry
Abstract
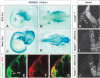
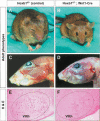
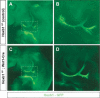
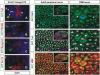
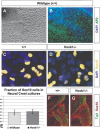
Formation of neuronal circuits in the head requires the coordinated development of neurons within the central nervous system (CNS) and neural crest-derived peripheral target tissues. Hoxb1, which is expressed throughout rhombomere 4 (r4), has been shown to be required for the specification of facial branchiomotor neuron progenitors that are programmed to innervate the muscles of facial expression. In this study, we have uncovered additional roles for Hoxb1-expressing cells in the formation and maintenance of the VIIth cranial nerve circuitry. By conditionally deleting the Hoxb1 locus in neural crest, we demonstrate that Hoxb1 is also required in r4-derived neural crest to facilitate and maintain formation of the VIIth nerve circuitry. Genetic lineage analysis revealed that a significant population of r4-derived neural crest is fated to generate glia that myelinate the VIIth cranial nerve. Neural crest cultures show that the absence of Hoxb1 function does not appear to affect overall glial progenitor specification, suggesting that a later glial function is critical for maintenance of the VIIth nerve. Taken together, these results suggest that the molecular program governing the development and maintenance of the VIIth cranial nerve is dependent upon Hoxb1, both in the neural crest-derived glia and in the facial branchiomotor neurons.
Figures




References
-
- Arenkiel B.R., Gaufo, G.O., and Capecchi, M.R. 2003. Hoxb1 neural crest preferentially form glia of the PNS. Dev. Dyn. 227: 379-386. - PubMed
-
- Arber S., Ladle, D.R., Lin, J.H., Frank, E., and Jessell, T.M. 2000. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101: 485-498. - PubMed
-
- Chai Y., Jiang, X., Ito, Y., Bringas Jr., P., Han, J., Rowitch, D.H., Soriano, P., McMahon, A.P., and Sucov, H.M. 2000. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127: 1671-1679. - PubMed
-
- Cooper K.L., Leisenring, W.M., and Capecchi, M.R. 2003. Autonomous and nonautonomous functions for Hox/Pbx in branchiomotor neuron development. Dev. Biol. 253: 200-213. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
