Synergistic transcription activation by Maf and Sox and their subnuclear localization are disrupted by a mutation in Maf that causes cataract
- PMID: 15199128
- PMCID: PMC480896
- DOI: 10.1128/MCB.24.13.5694-5709.2004
Synergistic transcription activation by Maf and Sox and their subnuclear localization are disrupted by a mutation in Maf that causes cataract
Abstract
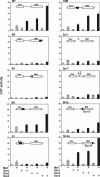
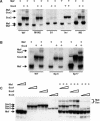
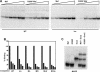
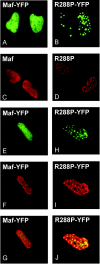
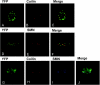
Crystallin genes are selectively expressed during lens development. Maf and Sox family proteins synergistically enhanced gammaF-crystallin promoter activity in a lens cell line. Mutational analysis of the gammaF-crystallin promoter identified a composite regulatory element containing nonconsensus Maf and Sox recognition sequences. Mutations in these recognition sequences or changes in their spacing eliminated synergistic transcription activation. The transcriptional synergy was also affected by changes in the orientation of the Maf recognition sequence that had no detectable effect on binding affinity. The interaction between Maf and Sox proteins was visualized in living cells by bimolecular fluorescence complementation analysis. The N-terminal region of Maf mediated the interaction with Sox proteins in cells. Synergistic transcription activation required the N-terminal region of Maf as well as the ancillary DNA binding domain and the unique portion of the basic region that mediate specific recognition of the gammaF-crystallin promoter element. A mutation in the ancillary DNA binding domain of Maf (R288P) that has been shown to cause cataract eliminated the transcriptional activity of Maf but had no detectable effect on DNA binding in vitro. Whereas wild-type Maf was uniformly distributed in the nucleoplasm, R288P Maf was enriched in nuclear foci. Cajal bodies and gemini of coiled bodies were closely associated with the foci occupied by R288P Maf. Wild-type Maf formed complexes with Sox proteins in the nucleoplasm, whereas R288P Maf recruited Sox proteins as well as other interaction partners to the nuclear foci. The mislocalization of normal cellular proteins to these foci provides a potential explanation for the dominant disease phenotype of the R288P mutation in Maf.
Figures






References
-
- Ambrosetti, D. C., H. R. Scholer, L. Dailey, and C. Basilico. 2000. Modulation of the activity of multiple transcriptional activation domains by the DNA binding domains mediates the synergistic action of Sox2 and Oct-3 on the fibroblast growth factor-4 enhancer. J. Biol. Chem. 275:23387-23397. - PubMed
-
- Brady, J. P., D. Garland, Y. Duglas-Tabor, W. G. Robison, Jr., A. Groome, and E. F. Wawrousek. 1997. Targeted disruption of the mouse alpha A-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc. Natl. Acad. Sci. USA 94:884-889. - PMC - PubMed
-
- Chen, Q., D. H. Dowhan, D. Liang, D. D. Moore, and P. A. Overbeek. 2002. CREB-binding protein/p300 co-activation of crystallin gene expression. J. Biol. Chem. 277:24081-24089. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
