DNA damage is a prerequisite for p53-mediated proteasomal degradation of HIF-1alpha in hypoxic cells and downregulation of the hypoxia marker carbonic anhydrase IX
- PMID: 15199132
- PMCID: PMC480909
- DOI: 10.1128/MCB.24.13.5757-5766.2004
DNA damage is a prerequisite for p53-mediated proteasomal degradation of HIF-1alpha in hypoxic cells and downregulation of the hypoxia marker carbonic anhydrase IX
Abstract
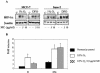
We investigated the relationship between the tumor suppressor p53 and the hypoxia-inducible factor-1 (HIF-1)-dependent expression of the hypoxia marker, carbonic anhydrase IX (CAIX). MCF-7 (wt p53) and Saos-2 (p53-null) cells displayed similar induction of CAIX expression and CA9 promoter activity under hypoxic conditions. Activation of p53 by the DNA damaging agent mitomycin C (MC) was accompanied by a potent repression of CAIX expression and the CA9 promoter in MCF-7 but not in Saos-2 cells. The activated p53 mediated increased proteasomal degradation of HIF-1alpha protein, resulting in considerably lower steady-state levels of HIF-1alpha protein in hypoxic MCF-7 cells but not in Saos-2 cells. Overexpression of HIF-1alpha relieved the MC-induced repression in MCF-7 cells, confirming regulation at the HIF-1alpha level. Similarly, CA9 promoter activity was downregulated by MC in HCT 116 p53(+/+) but not the isogenic p53(-/-) cells. Activated p53 decreased HIF-1alpha protein levels by accelerated proteasome-dependent degradation without affecting significantly HIF-1alpha transcription. In summary, our results demonstrate that the presence of wtp53 under hypoxic conditions has an insignificant effect on the stabilization of HIF-1alpha protein and HIF-1-dependent expression of CAIX. However, upon activation by DNA damage, wt p53 mediates an accelerated degradation of HIF-1alpha protein, resulting in reduced activation of CA9 transcription and, correspondingly, decreased levels of CAIX protein. A model outlining the quantitative relationship between p53, HIF-1alpha, and CAIX is presented.
Figures




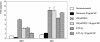

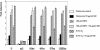

Similar articles
-
Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3'-kinase.Cancer Res. 2002 Aug 1;62(15):4469-77. Cancer Res. 2002. PMID: 12154057
-
Src induces expression of carbonic anhydrase IX via hypoxia-inducible factor 1.Oncol Rep. 2010 Mar;23(3):869-74. Oncol Rep. 2010. PMID: 20127031
-
Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: A one transcription factor (HIF-1) show?Biochim Biophys Acta. 2009 Apr;1795(2):162-72. doi: 10.1016/j.bbcan.2009.01.001. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19344680 Free PMC article. Review.
-
Hypoxia induced CA9 inhibitory targeting by two different sulfonamide derivatives including acetazolamide in human glioblastoma.Bioorg Med Chem. 2013 Jul 1;21(13):3949-57. doi: 10.1016/j.bmc.2013.03.068. Epub 2013 Apr 12. Bioorg Med Chem. 2013. PMID: 23706268
-
Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target.Cell Cycle. 2004 Feb;3(2):164-7. Cell Cycle. 2004. PMID: 14712082 Review.
Cited by
-
Complex regulation of the transactivation function of hypoxia-inducible factor-1 alpha by direct interaction with two distinct domains of the CREB-binding protein/p300.J Biol Chem. 2010 Jan 22;285(4):2601-9. doi: 10.1074/jbc.M109.021824. Epub 2009 Oct 30. J Biol Chem. 2010. PMID: 19880525 Free PMC article.
-
Reciprocal influence of the p53 and the hypoxic pathways.Cell Death Dis. 2011 May 26;2(5):e164. doi: 10.1038/cddis.2011.48. Cell Death Dis. 2011. PMID: 21614094 Free PMC article. Review.
-
Acne Transcriptomics: Fundamentals of Acne Pathogenesis and Isotretinoin Treatment.Cells. 2023 Nov 10;12(22):2600. doi: 10.3390/cells12222600. Cells. 2023. PMID: 37998335 Free PMC article. Review.
-
Differential carbonic anhydrase activities control EBV-induced B-cell transformation and lytic cycle reactivation.PLoS Pathog. 2024 Mar 26;20(3):e1011998. doi: 10.1371/journal.ppat.1011998. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38530845 Free PMC article.
-
Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells.Nano Lett. 2011 Jul 13;11(7):2796-803. doi: 10.1021/nl2011214. Epub 2011 Jun 9. Nano Lett. 2011. PMID: 21657258 Free PMC article.
References
-
- Achison, M., and T. R. Hupp. 2003. Hypoxia attenuates the p53 response to cellular damage. Oncogene 22:3431-3440. - PubMed
-
- Agani, F., D. G. Kirsch, S. L. Friedman, M. B. Kastan, and G. L. Semenza. 1997. p53 does not repress hypoxia-induced transcription of the vascular endothelial growth factor gene. Cancer Res. 57:4474-4477. - PubMed
-
- Airley, R. E., J. Loncaster, J. A. Raleigh, A. L. Harris, S. E. Davidson, R. D. Hunter, C. M. L. West, and I. J. Stratford. 2003. Glut-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int. J. Cancer 104:85-91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
