Human box H/ACA pseudouridylation guide RNA machinery
- PMID: 15199136
- PMCID: PMC480876
- DOI: 10.1128/MCB.24.13.5797-5807.2004
Human box H/ACA pseudouridylation guide RNA machinery
Abstract
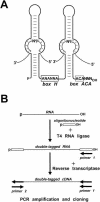
Pseudouridine, the most abundant modified nucleoside in RNA, is synthesized by posttranscriptional isomerization of uridines. In eukaryotic RNAs, site-specific synthesis of pseudouridines is directed primarily by box H/ACA guide RNAs. In this study, we have identified 61 novel putative pseudouridylation guide RNAs by construction and characterization of a cDNA library of human box H/ACA RNAs. The majority of the new box H/ACA RNAs are predicted to direct pseudouridine synthesis in rRNAs and spliceosomal small nuclear RNAs. We can attribute RNA-directed modification to 79 of the 97 pseudouridylation sites present in the human 18S, 5.8S, and 28S rRNAs and to 11 of the 21 pseudouridines reported for the U1, U2, U4, U5, and U6 spliceosomal RNAs. We have also identified 12 novel box H/ACA RNAs which lack apparent target pseudouridines in rRNAs and small nuclear RNAs. These putative guide RNAs likely function in the pseudouridylation of some other types of cellular RNAs, suggesting that RNA-guided pseudouridylation is more general than assumed before. The genomic organization of the new box H/ACA RNA genes indicates that in human cells, all box H/ACA pseudouridylation guide RNAs are processed from introns of pre-mRNA transcripts which either encode a protein product or lack protein-coding capacity.
Figures

References
-
- Agris, P. F. 1996. The importance of being modified: roles of modified nucleosides and Mg2+ in RNA structure and function. Prog. Nucleic Acids Res. Mol. Biol. 53:79-129. - PubMed
-
- Amaldi, F., and P. Pierandrei-Amaldi. 1997. TOP genes: a translationally controlled class of genes including those coding for ribosomal proteins. Prog. Mol. Subcell. Biol. 18:1-17. - PubMed
-
- Bakin, A., and J. Ofengand. 1993. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry 32:9754-9762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
