CCR4-associated factor CAF1 is an essential factor for spermatogenesis
- PMID: 15199137
- PMCID: PMC480892
- DOI: 10.1128/MCB.24.13.5808-5820.2004
CCR4-associated factor CAF1 is an essential factor for spermatogenesis
Abstract
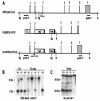
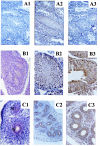
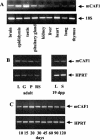
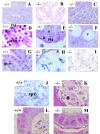
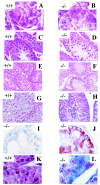
The CCR4-associated protein CAF1 has been demonstrated to play several roles in the control of transcription and of mRNA decay. To gain further insight into its physiological function, we generated CAF1-deficient mice. They are viable, healthy, and normal in appearance; however, mCAF1(-/-) male mice are sterile. The crossing of mCAF1(+/-) mice gave a Mendelian ratio of mCAF1(+/+), mCAF1(+/-), and mCAF1(-/-) pups, indicating that haploid mCAF1-deficient germ cells differentiate normally. The onset of the defect occurs during the first wave of spermatogenesis at 19 to 20 days after birth, during progression of pachytene spermatocytes to haploid spermatids and spermatozoa. Early disruption of spermatogenesis was evidenced by Sertoli cell vacuolization and tubular disorganization. The most mature germ cells were the most severely depleted, but progressively all germ cells were affected, giving Sertoli cell-only tubes, large interstitial spaces, and small testes. This phenotype could be linked to a defect(s) in germ cells and/or to inadequate Sertoli cell function, leading to seminiferous tubule disorganization and finally to a total disappearance of germ cells. The mCAF1-deficient mouse provides a new model of failed spermatogenesis in the adult that may be relevant to some cases of human male sterility.
Figures



References
-
- Bekelheide, K. (ed.). 1993. Sertoli cell toxicants in Sertoli cells. Cache River Press, Clearwater, Fla.
-
- Ben Sasson, S. A., Y. Sherman, and Y. Gavrieli. 1995. Identification of dying cells—in situ staining. Methods Cell Biol. 46:29-39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
