Development of a functional skin matrix requires deposition of collagen V heterotrimers
- PMID: 15199158
- PMCID: PMC480903
- DOI: 10.1128/MCB.24.13.6049-6057.2004
Development of a functional skin matrix requires deposition of collagen V heterotrimers
Abstract
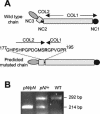
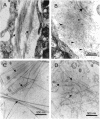
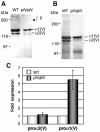
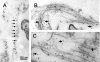
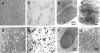
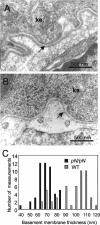
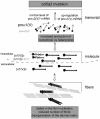
Collagen V is a minor component of the heterotypic I/III/V collagen fibrils and the defective product in most cases of classical Ehlers Danlos syndrome (EDS). The present study was undertaken to elucidate the impact of collagen V mutations on skin development, the most severely affected EDS tissues, using mice harboring a targeted deletion of the alpha2(V) collagen gene (Col5a2). Contrary to the original report, our studies indicate that the Col5a2 deletion (a.k.a. the pN allele) represents a functionally null mutation that affects matrix assembly through a complex sequence of events. First the mutation impairs assembly and/or secretion of the alpha1(V)(2)alpha2(V) heterotrimer with the result that the alpha1(V) homotrimer is the predominant species deposited into the matrix. Second, the alpha1(V) homotrimer is excluded from incorporation into the heterotypic collagen fibrils and this in turn severely impairs matrix organization. Third, the mutant matrix stimulates a compensatory loop by the alpha1(V) collagen gene that leads to additional deposition of alpha1(V) homotrimers. These data therefore underscore the importance of the collagen V heterotrimer in dermal fibrillogenesis. Furthermore, reduced thickness of the basement membranes underlying the epidermis and increased apoptosis of the stromal fibroblasts in pN/pN skin strongly indicate additional roles of collagen V in the development of a functional skin matrix.
Figures
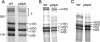
References
-
- Andrikopoulos, K., X. Liu, D. R. Keene, R. Jaenisch, and F. Ramirez. 1995. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat. Genet. 9:31-36. - PubMed
-
- Birk, D. E. 2001. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 32:223-237. - PubMed
-
- Birk, D. E., J. M. Fitch, J. P. Babiarz, K. J. Doane, and T. F. Linsenmayer. 1990. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 95:649-657. - PubMed
-
- Birk, D. E., J. M. Fitch, and T. F. Linsenmayer. 1986. Organization of collagen types I and V in the embryonic chicken cornea. Investig. Ophthalmol. Vis. Sci. 27:1470-1477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
