Rapid preparation of RNA samples for NMR spectroscopy and X-ray crystallography
- PMID: 15199176
- PMCID: PMC434460
- DOI: 10.1093/nar/gnh081
Rapid preparation of RNA samples for NMR spectroscopy and X-ray crystallography
Abstract
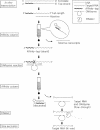
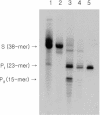
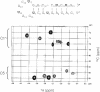
Knowledge of the three-dimensional structures of RNA and its complexes is important for understanding the molecular mechanism of RNA recognition by proteins or ligands. Enzymatic synthesis using T7 bacteriophage RNA polymerase is used to prepare samples for NMR spectroscopy and X-ray crystallography. However, this run-off transcription method results in heterogeneity at the RNA 3-terminus. For structural studies, RNA purification requires a single nucleotide resolution. Usually PAGE purification is used, but it is tedious, time-consuming and cost ineffective. To overcome these problems in high-throughput RNA synthesis, we devised a method of RNA preparation that uses trans-acting DNAzyme and sequence-specific affinity column chromatography. A tag sequence is added at the 3' end of RNA, and the tagged RNA is picked out using an affinity column that contains the complementary DNA sequence. The 3' end tag is then removed by sequence-specific cleavage using trans-acting DNAzyme, the arm lengths of which are optimized for turnover number. This purification method is simpler and faster than the conventional method.
Figures

References
-
- Milligan J.F. and Uhlenbeck. O.C. (1989) Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol., 180, 51–62. - PubMed
-
- Rasmussen S.R., Larsen,M.R. and Rasmussen,S.E. (1991) Covalent immobilization of DNA onto polystyrene microwells: the molecules are only bound at the 5′ end. Anal. Biochem., 198, 138–142. - PubMed
-
- Lee G.-Y., Chen,C.-H., Wang,T.-H. and Lee,W.-C. (2003) Affinity chromatography of DNA on nonporous copolymerized particles of styrene and glycidyl methacrylate with immobilized polynucleotide. Anal. Biochem., 312, 235–241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

