Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin
- PMID: 15199181
- PMCID: PMC470754
- DOI: 10.1073/pnas.0401104101
Identification of a domain within the multifunctional Vibrio cholerae RTX toxin that covalently cross-links actin
Abstract
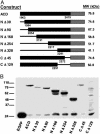
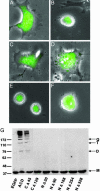
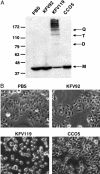
The Gram-negative pathogen Vibrio cholerae causes diarrheal disease through the export of enterotoxins. The V. cholerae RTX toxin was previously identified and characterized by its ability to round human laryngeal epithelial (HEp-2) cells. Further investigation determined that cell rounding is caused by the depolymerization of actin stress fibers, through the unique mechanism of covalent actin cross-linking. In this study, we identify a domain within the full-length RTX toxin that is capable of mediating the cross-linking reaction when transiently expressed within eukaryotic cells. A structure/function analysis of the actin cross-linking domain (ACD) reveals that a 412-aa, or a 47.8-kDa, region is essential for cross-linking activity. When this domain is deleted from the full-length toxin gene, actin cross-linking, but not cell rounding, is eliminated, indicating that this toxin carries multiple dissociable activities. The ACD shares 59% amino acid identity with a hypothetical protein from V. cholerae, VC1416, and transient expression of the C-terminal domain of VC1416 also results in actin cross-linking in eukaryotic cells. The presence of this second ACD linked to an Rhs-like element suggests that V. cholerae acquired the domain by horizontal gene transfer and the ACD was inserted into the RTX toxin by gene duplication through the evolution of V. cholerae.
Figures
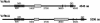
Similar articles
-
The Actin cross-linking domain of the Vibrio cholerae RTX toxin directly catalyzes the covalent cross-linking of actin.J Biol Chem. 2006 Oct 27;281(43):32366-74. doi: 10.1074/jbc.M605275200. Epub 2006 Sep 5. J Biol Chem. 2006. PMID: 16954226 Free PMC article.
-
In vivo covalent cross-linking of cellular actin by the Vibrio cholerae RTX toxin.EMBO J. 2000 Oct 16;19(20):5315-23. doi: 10.1093/emboj/19.20.5315. EMBO J. 2000. PMID: 11032799 Free PMC article.
-
Characterization of the enzymatic activity of the actin cross-linking domain from the Vibrio cholerae MARTX Vc toxin.J Biol Chem. 2008 Jan 4;283(1):445-452. doi: 10.1074/jbc.M703910200. Epub 2007 Oct 20. J Biol Chem. 2008. PMID: 17951576 Free PMC article.
-
Pathogenic Mechanisms of Actin Cross-Linking Toxins: Peeling Away the Layers.Curr Top Microbiol Immunol. 2017;399:87-112. doi: 10.1007/82_2016_22. Curr Top Microbiol Immunol. 2017. PMID: 27858184 Review.
-
[Toxins of Vibrio cholerae].Mol Gen Mikrobiol Virusol. 2005;(1):7-18. Mol Gen Mikrobiol Virusol. 2005. PMID: 15790027 Review. Russian.
Cited by
-
The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus.Infect Immun. 2007 Jul;75(7):3282-9. doi: 10.1128/IAI.00045-07. Epub 2007 Apr 16. Infect Immun. 2007. PMID: 17438022 Free PMC article.
-
The Vibrio cholerae MARTX toxin silences the inflammatory response to cytoskeletal damage before inducing actin cytoskeleton collapse.Sci Signal. 2020 Jan 14;13(614):eaaw9447. doi: 10.1126/scisignal.aaw9447. Sci Signal. 2020. PMID: 31937566 Free PMC article.
-
MARTX toxins as effector delivery platforms.Pathog Dis. 2015 Dec;73(9):ftv092. doi: 10.1093/femspd/ftv092. Epub 2015 Oct 15. Pathog Dis. 2015. PMID: 26472741 Free PMC article. Review.
-
Structure-function analysis of inositol hexakisphosphate-induced autoprocessing of the Vibrio cholerae multifunctional autoprocessing RTX toxin.J Biol Chem. 2008 Aug 29;283(35):23656-64. doi: 10.1074/jbc.M803334200. Epub 2008 Jun 30. J Biol Chem. 2008. PMID: 18591243 Free PMC article.
-
Inactivation of small Rho GTPases by the multifunctional RTX toxin from Vibrio cholerae.Cell Microbiol. 2007 May;9(5):1324-35. doi: 10.1111/j.1462-5822.2006.00876.x. Cell Microbiol. 2007. PMID: 17474905 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

