A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin
- PMID: 15199184
- PMCID: PMC454183
- DOI: 10.1073/pnas.0402829101
A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin
Abstract
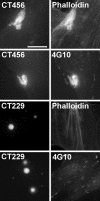
The obligate intracellular bacterium Chlamydia trachomatis rapidly induces its own entry into host cells. Initial attachment is mediated by electrostatic interactions to heparan sulfate moieties on the host cell, followed by irreversible binding to an unknown secondary receptor. This secondary binding leads to the recruitment of actin to the site of attachment, formation of an actin-rich, pedestal-like structure, and finally internalization of the bacteria. How chlamydiae induce this process is unknown. We have identified a high-molecular-mass tyrosine-phosphorylated protein that is rapidly phosphorylated on attachment to the host cell. Immunoelectron microscopy studies revealed that this tyrosine-phosphorylated protein is localized to the cytoplasmic face of the plasma membrane at the site of attachment of surface-associated chlamydiae. The phosphoprotein was isolated by immunoprecipitation with the antiphosphotyrosine antibody 4G10 and identified as the chlamydial protein CT456, a hypothetical protein with unknown function. The chlamydial protein (Tarp) appears to be translocated into the host cell by type III secretion because it is exported in a Yersinia heterologous expression assay. Phosphotyrosine signaling across the plasma membrane preceded the recruitment of actin to the site of chlamydial attachment and may represent the initial signal transduced from pathogen to the host cell. These results suggest that C. trachomatis internalization is mediated by a chlamydial type III-secreted effector protein.
Figures





Comment in
-
Tarp and Arp: How Chlamydia induces its own entry.Proc Natl Acad Sci U S A. 2004 Jul 6;101(27):9947-8. doi: 10.1073/pnas.0403633101. Epub 2004 Jun 29. Proc Natl Acad Sci U S A. 2004. PMID: 15226494 Free PMC article. No abstract available.
References
-
- Schachter, J. (1999) in Chlamydia: Intracellular Biology, Pathogenesis, and Immunity, ed. Stephens, R. S. (Am. Soc. Microbiol., Washington, DC), pp. 139-169.
-
- Hackstadt, T. (1999) in Chlamydia: Intracellular Biology, Pathogenesis, and Immunity., ed. Stephens, R. S. (Am. Soc. Microbiol., Washington, DC), pp. 101-138.
-
- Zhang, J. P. & Stephens, R. S. (1992) Cell 69, 861-869. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

