Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice
- PMID: 15199411
- PMCID: PMC420503
- DOI: 10.1172/JCI20369
Novel targeted deregulation of c-Myc cooperates with Bcl-X(L) to cause plasma cell neoplasms in mice
Abstract
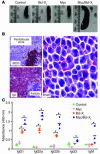
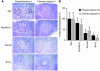
Deregulated expression of both Myc and Bcl-X(L) are consistent features of human plasma cell neoplasms (PCNs). To investigate whether targeted expression of Myc and Bcl-X(L) in mouse plasma cells might lead to an improved model of human PCN, we generated Myc transgenics by inserting a single-copy histidine-tagged mouse Myc gene, Myc(His), into the mouse Ig heavy-chain Calpha locus. We also generated Bcl-X(L) transgenic mice that contain a multicopy Flag-tagged mouse Bcl-x(Flag) transgene driven by the mouse Ig kappa light-chain 3' enhancer. Single-transgenic Bcl-X(L) mice remained tumor free by 380 days of age, whereas single-transgenic Myc mice developed B cell tumors infrequently (4 of 43, 9.3%). In contrast, double-transgenic Myc/Bcl-X(L) mice developed plasma cell tumors with short onset (135 days on average) and full penetrance (100% tumor incidence). These tumors produced monoclonal Ig, infiltrated the bone marrow, and contained elevated amounts of Myc(His) and Bcl-X(L)(Flag) proteins compared with the plasma cells that accumulated in large numbers in young tumor-free Myc/Bcl-X(L) mice. Our findings demonstrate that the enforced expression of Myc and Bcl-X(L) by Ig enhancers with peak activity in plasma cells generates a mouse model of human PCN that recapitulates some features of human multiple myeloma.
Figures




References
-
- Radl J. Multiple myeloma and related disorders. Lessons from an animal model. Pathol. Biol. (Paris). 1999;47:109–114. - PubMed
-
- Potter M. Experimental plasmacytomagenesis in mice. Hematol. Oncol. Clin. North Am. 1997;11:323–347. - PubMed
-
- Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–2913. - PubMed
-
- Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94:3576–3582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

