Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas
- PMID: 15199412
- PMCID: PMC420505
- DOI: 10.1172/JCI20513
Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas
Abstract
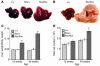
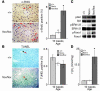
PTEN is a tumor suppressor gene mutated in many human cancers, and its expression is reduced or absent in almost half of hepatoma patients. We used the Cre-loxP system to generate a hepatocyte-specific null mutation of Pten in mice (AlbCrePten(flox/flox) mice). AlbCrePten(flox/flox) mice showed massive hepatomegaly and steatohepatitis with triglyceride accumulation, a phenotype similar to human nonalcoholic steatohepatitis. Adipocyte-specific genes were induced in mutant hepatocytes, implying adipogenic-like transformation of these cells. Genes involved in lipogenesis and beta-oxidation were also induced, possibly as a result of elevated levels of the transactivating factors PPARgamma and SREBP1c. Importantly, the loss of Pten function in the liver led to tumorigenesis, with 47% of AlbCrePten(flox/flox) livers developing liver cell adenomas by 44 weeks of age. By 74-78 weeks of age, 100% of AlbCrePten(flox/flox) livers showed adenomas and 66% had hepatocellular carcinomas. AlbCrePten(flox/flox) mice also showed insulin hypersensitivity. In vitro, AlbCrePten(flox/flox) hepatocytes were hyperproliferative and showed increased hyperoxidation with abnormal activation of protein kinase B and MAPK. Pten is thus an important regulator of lipogenesis, glucose metabolism, hepatocyte homeostasis, and tumorigenesis in the liver.
Figures
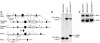



Similar articles
-
Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation.Cancer Res. 2003 Feb 1;63(3):674-81. Cancer Res. 2003. PMID: 12566313
-
PTEN modulates insulin-like growth factor II (IGF-II)-mediated signaling; the protein phosphatase activity of PTEN downregulates IGF-II expression in hepatoma cells.FEBS Lett. 2003 Jun 19;545(2-3):203-8. doi: 10.1016/s0014-5793(03)00535-0. FEBS Lett. 2003. PMID: 12804776
-
Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients.Cancer Res. 2006 Sep 1;66(17):8389-96. doi: 10.1158/0008-5472.CAN-05-4627. Cancer Res. 2006. PMID: 16951148
-
[Tumor suppressor gene PTEN and non-alcoholic steatohepatitis (NASH)].Nihon Rinsho. 2005 Aug;63(8):1475-83. Nihon Rinsho. 2005. PMID: 16101243 Review. Japanese.
-
PTEN in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and cancer.Dig Dis. 2010;28(1):236-46. doi: 10.1159/000282095. Epub 2010 May 7. Dig Dis. 2010. PMID: 20460918 Review.
Cited by
-
Liver carcinogenesis: rodent models of hepatocarcinoma and cholangiocarcinoma.Dig Liver Dis. 2013 Jun;45(6):450-9. doi: 10.1016/j.dld.2012.10.008. Epub 2012 Nov 22. Dig Liver Dis. 2013. PMID: 23177172 Free PMC article. Review.
-
Transformation of SOX9+ cells by Pten deletion synergizes with steatotic liver injury to drive development of hepatocellular and cholangiocarcinoma.Sci Rep. 2021 Jun 3;11(1):11823. doi: 10.1038/s41598-021-90958-1. Sci Rep. 2021. PMID: 34083580 Free PMC article.
-
Overexpression of GRIM-19, a mitochondrial respiratory chain complex I protein, suppresses hepatocellular carcinoma growth.Int J Clin Exp Pathol. 2014 Oct 15;7(11):7497-507. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25550785 Free PMC article.
-
Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma.Cancer Res. 2013 Aug 1;73(15):4722-31. doi: 10.1158/0008-5472.CAN-12-3797. Epub 2013 Jun 7. Cancer Res. 2013. PMID: 23749645 Free PMC article.
-
Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.World J Gastroenterol. 2012 May 21;18(19):2300-8. doi: 10.3748/wjg.v18.i19.2300. World J Gastroenterol. 2012. PMID: 22654421 Free PMC article. Review.
References
-
- Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. - PubMed
-
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. - PubMed
-
- Yao YJ, et al. PTEN/MMAC1 mutations in hepatocellular carcinomas. Oncogene. 1999;18:3181–3185. - PubMed
-
- Hu TH, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929–1940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

