Assisted reproductive technologies in rhesus macaques
- PMID: 15200674
- PMCID: PMC441410
- DOI: 10.1186/1477-7827-2-37
Assisted reproductive technologies in rhesus macaques
Abstract
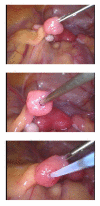
The assisted reproductive technologies (ARTs) have been used in the production of rhesus monkey offspring at the Oregon National Primate Research Center (ONPRC) and that experience is summarized here. Additionally these technologies serve as a source of oocytes/embryos for monozygotic twinning, embryonic stem (ES) cell derivation and cloning. High fertilization efficiencies were realized with conventional insemination or following the use of intracytoplasmic sperm injection (ICSI) and approximately 50% of the resulting embryos grew in vitro to blastocysts. Both fresh and frozen sperm were employed in fertilization by ICSI and the resulting embryos could be low temperature stored for subsequent thawing and transfer when a synchronized recipient female was available or after shipment to another facility. Following the transfer of up to 3 embryos, an overall pregnancy rate of 30% was achieved with increasing rates dependent upon the number of embryos transferred. Singleton pregnancy outcomes following the transfer of ART produced embryos were similar to those observed in a control group of animals in the timed mated breeding colony at ONPRC. ICSI produced embryos were used in efforts to create monozygotic twins by blastomere separation or blastocyst splitting. While pregnancies were achieved following the transfer of demi-embryos, only one was a twin and it was lost to spontaneous abortion. ICSI produced embryos have also served as the source of blastocysts for the derivation of embryonic stem cells. These pluripotent cells hold potential for cell based therapies and we consider the monkey an important translational model in which to evaluate safety, efficacy and feasibility of regenerative medicine approaches based on the transplantation of stem cell-derived progeny. Finally, efforts to produce genetically-identical monkeys by nuclear transfer have been briefly summarized.
Figures



Similar articles
-
Use of assisted reproductive technologies in the propagation of rhesus macaque offspring.Biol Reprod. 2004 Aug;71(2):486-93. doi: 10.1095/biolreprod.103.025932. Epub 2004 Mar 24. Biol Reprod. 2004. PMID: 15044263
-
Monozygotic twinning in rhesus monkeys by manipulation of in vitro-derived embryos.Biol Reprod. 2002 May;66(5):1449-55. doi: 10.1095/biolreprod66.5.1449. Biol Reprod. 2002. PMID: 11967209
-
Neonatal behavior and infant cognitive development in rhesus macaques produced by assisted reproductive technologies.Dev Psychobiol. 2006 Apr;48(3):243-65. doi: 10.1002/dev.20132. Dev Psychobiol. 2006. PMID: 16568416
-
Multi-chorionic pregnancies following single embryo transfer at the blastocyst stage: a case series and review of the literature.J Assist Reprod Genet. 2018 Dec;35(12):2109-2117. doi: 10.1007/s10815-018-1329-8. Epub 2018 Oct 25. J Assist Reprod Genet. 2018. PMID: 30362060 Free PMC article. Review.
-
Guidelines for the number of embryos to transfer following in vitro fertilization No. 182, September 2006.Int J Gynaecol Obstet. 2008 Aug;102(2):203-16. doi: 10.1016/j.ijgo.2008.01.007. Int J Gynaecol Obstet. 2008. PMID: 18773532 Review.
Cited by
-
Efficient reproduction of cynomolgus monkey using pronuclear embryo transfer technique.Proc Natl Acad Sci U S A. 2008 Sep 2;105(35):12956-60. doi: 10.1073/pnas.0805639105. Epub 2008 Aug 25. Proc Natl Acad Sci U S A. 2008. PMID: 18725640 Free PMC article.
-
Transcriptome profiling of individual rhesus macaque oocytes and preimplantation embryos.Biol Reprod. 2017 Sep 1;97(3):353-364. doi: 10.1093/biolre/iox114. Biol Reprod. 2017. PMID: 29025079 Free PMC article.
-
Leveraging human genomic information to identify nonhuman primate sequences for expression array development.BMC Genomics. 2005 Nov 15;6:160. doi: 10.1186/1471-2164-6-160. BMC Genomics. 2005. PMID: 16288651 Free PMC article.
-
Oxidative damage to rhesus macaque spermatozoa results in mitotic arrest and transcript abundance changes in early embryos.Biol Reprod. 2013 Sep 27;89(3):72. doi: 10.1095/biolreprod.113.110981. Print 2013 Sep. Biol Reprod. 2013. PMID: 23904511 Free PMC article.
-
Generation of Rhesus Macaque Embryos with Expanded CAG Trinucleotide Repeats in the Huntingtin Gene.Cells. 2024 May 13;13(10):829. doi: 10.3390/cells13100829. Cells. 2024. PMID: 38786052 Free PMC article.
References
-
- Ouhibi N, Zelinski-Wooten MB, Thomson JA, Wolf DP. Assisted fertilization and nuclear transfer in nonhuman primates. In: Wolf DP, Zelinski-Wooten M, editor. In Assisted Fertilization and Nuclear Transfer in Mammals. New Jersey: Humana Press Totowa; 2001. pp. 253–284.
-
- Tarlatzis BC, Bili H. Survey on intracytoplasmic sperm injection: report from the ESHRE ICSI Task Force. European Society of Human Reproduction and Embryology. Hum Reprod. 1998;13:165–177. - PubMed
-
- Bonduelle M, Wilikens A, Buysse A, Van Assche E, Wisanto A, Devroey P, Van Steirteghem AC, Liebaers I. Prospective follow-up study of 877 children born after intracytoplasmic sperm injection (ICSI), with ejaculated epididymal and testicular spermatozoa and after replacement of cryopreserved embryos obtained after ICSI. Hum Reprod. 1996;11:1558–1564. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

