Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity
- PMID: 15200679
- PMCID: PMC442134
- DOI: 10.1186/1477-7827-2-32
Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity
Abstract
Selection of the species-specific number of follicles that will develop and ovulate during the ovarian cycle can be overridden by increasing the levels of pituitary gonadotropin hormones, FSH and LH. During controlled ovarian stimulation (COS) in nonhuman primates for assisted reproductive technology (ART) protocols, the method of choice (but not the only method) has been the administration of exogenous gonadotropins, either of nonprimate or primate origin. Due to species-specificity of the primate LH (but not FSH) receptor, COS with nonprimate (e.g., PMSG) hormones can be attributed to their FSH activity. Elevated levels of FSH alone will produce large antral follicles containing oocytes capable of fertilization in vitro (IVF). However, there is evidence that LH, probably in lesser amounts, increases the rate of follicular development, reduces heterogeneity of the antral follicle pool, and improves the viability and rate of pre-implantation development of IVF-produced embryos. Since an endogenous LH surge typically does not occur during COS cycles (especially when a GnRH antagonist is added), a large dose of an LH-like hormone (i.e., hCG) may be given to reinitiate meiosis and produce fertilizable oocytes. Alternate approaches using exogenous LH (or FSH), or GnRH agonist to induce an endogenous LH surge, have received lesser attention. Current protocols will routinely yield dozens of large follicles with fertilizable eggs. However, limitations include non/poor-responding animals, heterogeneity of follicles (and presumably oocytes) and subsequent short luteal phases (limiting embryo transfer in COS cycles). However, the most serious limitation to further improvements and expanded use of COS protocols for ART is the lack of availability of nonhuman primate gonadotropins. Human, and even more so, nonprimate gonadotropins are antigenic in monkeys, which limits the number of COS cycles to as few as 1 (PMSG) or 3 (recombinant hCG) protocols in macaques. Production and access to sufficient supplies of nonhuman primate FSH, LH and CG would overcome this major hurdle.
Figures



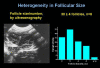
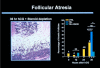
Similar articles
-
The role of gonadotropins in follicular development and their use in ovulation induction protocols for assisted reproduction.Eur J Contracept Reprod Health Care. 2000 Jun;5(2):157-67. doi: 10.1080/13625180008500389. Eur J Contracept Reprod Health Care. 2000. PMID: 10943580 Review.
-
Luteal granulosa cells from natural cycles are more capable of maintaining their viability, steroidogenic activity and LH receptor expression than those of stimulated IVF cycles.Hum Reprod. 2019 Feb 1;34(2):345-355. doi: 10.1093/humrep/dey353. Hum Reprod. 2019. PMID: 30520979
-
Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥35 years: a randomized controlled trial.Hum Reprod. 2015 May;30(5):1188-95. doi: 10.1093/humrep/dev038. Epub 2015 Mar 3. Hum Reprod. 2015. PMID: 25740882 Clinical Trial.
-
Induction of multiple follicular development by a single dose of long-acting recombinant follicle-Stimulating hormone (FSH-CTP, corifollitropin alfa) for controlled ovarian stimulation before in vitro fertilization.J Clin Endocrinol Metab. 2004 May;89(5):2062-70. doi: 10.1210/jc.2003-031766. J Clin Endocrinol Metab. 2004. PMID: 15126522 Clinical Trial.
-
[Medroxyprogesteron acetate use to block LH surge in oocyte donor stimulation].Ceska Gynekol. 2018 Winter;83(1):11-16. Ceska Gynekol. 2018. PMID: 29510633 Review. Czech.
Cited by
-
The usefulness of metaphase I oocytes in women who undergo controlled ovarian hyperstimulation for intracytoplasmic sperm injection.JBRA Assist Reprod. 2021 Feb 2;25(1):115-121. doi: 10.5935/1518-0557.20200062. JBRA Assist Reprod. 2021. PMID: 33021764 Free PMC article.
-
Long-term Hyperandrogenemia and/or Western-style Diet in Rhesus Macaque Females Impairs Preimplantation Embryogenesis.Endocrinology. 2022 Apr 1;163(4):bqac019. doi: 10.1210/endocr/bqac019. Endocrinology. 2022. PMID: 35192701 Free PMC article.
-
A successful pregnancy following recurrent implantation failure with clinical laboratory strategy.JBRA Assist Reprod. 2020 Oct 6;24(4):507-509. doi: 10.5935/1518-0557.20190093. JBRA Assist Reprod. 2020. PMID: 32401454 Free PMC article.
-
Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis.Mol Reprod Dev. 2014 Oct;81(10):928-45. doi: 10.1002/mrd.22413. Epub 2014 Sep 19. Mol Reprod Dev. 2014. PMID: 25242074 Free PMC article.
-
Use of controlled ovulation of the dominant follicle to assess oocyte maturation during natural menstrual cycles in rhesus macaques.Fertil Steril. 2007 Jun;87(6):1477-9. doi: 10.1016/j.fertnstert.2006.11.059. Epub 2007 Jan 25. Fertil Steril. 2007. PMID: 17258212 Free PMC article.
References
-
- Zeleznik AJ, Benyo DF. Control of follicular development, corpus luteum function, and the recognition of pregnancy in higher primates. In: KnobilE and NeillJD, editor. The Physiology of Reproduction. New York, Raven Press, Ltd.; 1994. pp. 751–782.
-
- Dukelow WR, Vengesa PN. Primate models for fertilization and early embryogenesis. In: BenirschkeK, editor. Primates The Road to Self-Sustaining Populations. New York, Springer-Verlag; 1986. pp. 445–461.
-
- Zeleznik AJ. The physiology and cell biology of follicle selection. ARTS in Action in Non-human Primates Post-conference Symposium Associated with the 2004 IETS Annual Meeting (held in Portland, OR, January 14-15) 2004.
-
- Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: The technique and its applications. Mol Reprod Dev. 1990;27:261–280. - PubMed
-
- Stouffer RL, Zelinski-Wooten MB, Aladin Chandrasekher Y, Wolf DP. Stimulation of follicle and oocyte development in macaques for IVF procedures. In: WolfDP, StoufferRL and BrennerRM, editor. In Vitro Fertilization and Embryo Transfer in Primates. New York, Springer-Verlag; 1993. pp. 124–141.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

