Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs
- PMID: 15200845
- PMCID: PMC3323075
- DOI: 10.3201/eid1004.030458
Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs
Abstract
Severe acute respiratory syndrome (SARS) is an infectious disease caused by a newly identified human coronavirus (SARS-CoV). Currently, no effective drug exists to treat SARS-CoV infection. In this study, we investigated whether a panel of commercially available antiviral drugs exhibit in vitro anti-SARS-CoV activity. A drug-screening assay that scores for virus-induced cytopathic effects on cultured cells was used. Tested were 19 clinically approved compounds from several major antiviral pharmacologic classes: nucleoside analogs, interferons, protease inhibitors, reverse transcriptase inhibitors, and neuraminidase inhibitors. Complete inhibition of cytopathic effects of SARS-CoV in culture was observed for interferon subtypes, b-1b, a-n1, a-n3, and human leukocyte interferon a. These findings support clinical testing of approved interferons for the treatment of SARS.
Figures

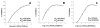
References
-
- World Health Organization. Acute respiratory syndrome in China—update 3: disease outbreak reported: Geneva: The Organization; 2003.
-
- Severe acute respiratory syndrome (SARS) and coronavirus testing—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:297–302. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
