Modeling the actions of beta-adrenergic signaling on excitation--contraction coupling processes
- PMID: 15201146
- PMCID: PMC1201510
- DOI: 10.1196/annals.1302.002
Modeling the actions of beta-adrenergic signaling on excitation--contraction coupling processes
Abstract
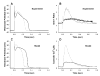
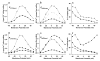
Activation of the beta-adrenergic (beta-AR) signaling pathway enhances cardiac function through protein kinase A (PKA)-mediated phosphorylation of target proteins involved in the process of excitation-contraction (EC) coupling. Experimental studies of the effects of beta-AR stimulation on EC coupling have yielded complex results, including increased, decreased, or unchanged EC coupling gain. In this study, we extend a previously developed model of the canine ventricular myocyte describing local control of sarcoplasmic reticulum (SR) calcium (Ca(2+)) release to include the effects of beta-AR stimulation. Incorporation of phosphorylation-dependent effects on model membrane currents and Ca(2+)-cycling proteins yields changes of action potential (AP) and Ca(2+) transients in agreement with those measured experimentally in response to the nonspecific beta-AR agonist isoproterenol (ISO). The model reproduces experimentally observed alterations in EC coupling gain in response to beta-AR agonists and predicts the specific roles of L-type Ca(2+) channel (LCC) and SR Ca(2+) release channel phosphorylation in altering the amplitude and shape of the EC coupling gain function. The model also indicates that factors that promote mode 2 gating of LCCs, such as beta-AR stimulation or activation of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII), may increase the probability of occurrence of early after-depolarizations (EADs), due to the random, long-duration opening of LCC gating in mode 2.
Figures
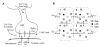
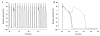
Similar articles
-
Polydatin modulates Ca(2+) handling, excitation-contraction coupling and β-adrenergic signaling in rat ventricular myocytes.J Mol Cell Cardiol. 2012 Nov;53(5):646-56. doi: 10.1016/j.yjmcc.2012.08.009. Epub 2012 Aug 19. J Mol Cell Cardiol. 2012. PMID: 22921781
-
Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart.Circ Res. 2010 Feb 5;106(2):317-27. doi: 10.1161/CIRCRESAHA.109.208272. Epub 2009 Nov 19. Circ Res. 2010. PMID: 19926875
-
Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase.Circ Res. 2007 Feb 16;100(3):391-8. doi: 10.1161/01.RES.0000258172.74570.e6. Epub 2007 Jan 18. Circ Res. 2007. PMID: 17234966
-
Epac in cardiac calcium signaling.J Mol Cell Cardiol. 2013 May;58:162-71. doi: 10.1016/j.yjmcc.2012.11.021. Epub 2012 Dec 7. J Mol Cell Cardiol. 2013. PMID: 23220153 Review.
-
Rhythmic Ca2+ oscillations drive sinoatrial nodal cell pacemaker function to make the heart tick.Ann N Y Acad Sci. 2005 Jun;1047:138-56. doi: 10.1196/annals.1341.013. Ann N Y Acad Sci. 2005. PMID: 16093492 Review.
Cited by
-
The force-frequency relationship: insights from mathematical modeling.Adv Physiol Educ. 2013 Mar;37(1):28-34. doi: 10.1152/advan.00072.2011. Adv Physiol Educ. 2013. PMID: 23471245 Free PMC article. Review.
-
Estimating ectopic beat probability with simplified statistical models that account for experimental uncertainty.PLoS Comput Biol. 2021 Oct 19;17(10):e1009536. doi: 10.1371/journal.pcbi.1009536. eCollection 2021 Oct. PLoS Comput Biol. 2021. PMID: 34665814 Free PMC article.
-
From mitochondrial ion channels to arrhythmias in the heart: computational techniques to bridge the spatio-temporal scales.Philos Trans A Math Phys Eng Sci. 2008 Sep 28;366(1879):3381-409. doi: 10.1098/rsta.2008.0112. Philos Trans A Math Phys Eng Sci. 2008. PMID: 18603526 Free PMC article. Review.
-
The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations.Biophys J. 2005 Jan;88(1):85-95. doi: 10.1529/biophysj.104.051508. Epub 2004 Oct 22. Biophys J. 2005. PMID: 15501946 Free PMC article.
-
A mathematical model of action potentials of mouse sinoatrial node cells with molecular bases.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H945-63. doi: 10.1152/ajpheart.00143.2010. Epub 2011 Jul 1. Am J Physiol Heart Circ Physiol. 2011. PMID: 21724866 Free PMC article.
References
-
- Xiao RP, et al. Recent advances in cardiac β2-adrenergic signal transduction. Circ Res. 1999;85:1092–1100. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. - PubMed
-
- Li Y, et al. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

