Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency
- PMID: 15201317
- PMCID: PMC6729318
- DOI: 10.1523/JNEUROSCI.1106-04.2004
Choline transporter 1 maintains cholinergic function in choline acetyltransferase haploinsufficiency
Abstract
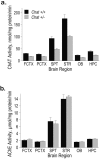
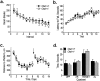
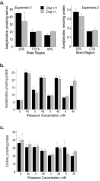
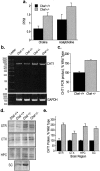
Choline acetyltransferase (ChAT), the enzyme that synthesizes the neurotransmitter acetylcholine (ACh), is thought to be present in kinetic excess in cholinergic neurons. The rate-limiting factor in ACh production is the provision of choline to ChAT. Cholinergic neurons are relatively unique in their expression of the choline transporter 1 (CHT1), which exhibits high-affinity for choline and catalyzes its uptake from the extracellular space to the neuron. Multiple lines of evidence indicate that the activity of CHT1 is a key determinant of choline supply for ACh synthesis. We examined the interaction of ChAT and ChT activity using mice heterozygous for a null mutation in the Chat gene (Chat+/-). In these mice, brain ChAT activity was reduced by 40-50% relative to the wild type, but brain ACh levels as well as ACh content and depolarization-evoked ACh release in hippocampal slices were normal. However, the amount of choline taken up by CHT1 and ACh synthesized de novo from choline transported by CHT1 in hippocampal slices, as well as levels of CHT1 mRNA in the septum and CHT1 protein in several regions of the CNS, were 50-100% higher in Chat+/- than in Chat+/+ mice. Thus, haploinsufficiency of ChAT leads to an increased expression of CHT1. Increased ChT activity may compensate for the reduced ChAT activity in Chat+/- mice, contributing to the maintenance of apparently normal cholinergic function as reflected by normal performance of these mice in several behavioral assays.
Figures
References
-
- Apparsundaram S, Ferguson SM, George Jr AL, Blakely RD (2000) Molecular cloning of a human, Hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun 276: 862-867. - PubMed
-
- Blusztajn JK, Wurtman RJ (1981) Choline biosynthesis by a preparation enriched in synaptosomes from rat brain. Nature 290: 417-418. - PubMed
-
- Blusztajn JK, Wurtman RJ (1983) Choline and cholinergic neurons. Science 221: 614-620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
