NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius
- PMID: 15201324
- PMCID: PMC6729325
- DOI: 10.1523/JNEUROSCI.1176-04.2004
NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius
Abstract
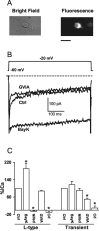
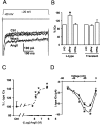
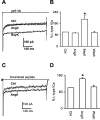
Angiotensin II (AngII), acting through angiotensin type 1 (AT1) receptors, exerts powerful effects on central autonomic networks regulating cardiovascular homeostasis and fluid balance; however, the mechanisms of AngII signaling in functionally defined central autonomic neurons have not been fully elucidated. In vascular cells, reactive oxygen species (ROS) generated by the enzyme NADPH oxidase play a major role in AngII signaling. Thus, we sought to determine whether NADPH oxidase is present in central autonomic neurons and, if so, whether NADPH oxidase-derived ROS are involved in the effects of AngII on these neurons. The present studies focused on the intermediate dorsomedial nucleus of the solitary tract (dmNTS) because this region receives autonomic afferents via the vagus nerve and is an important site of AngII actions. Using double-label immunoelectron microscopy, we found that the essential NADPH oxidase subunit gp91phox is present in somatodendric and axonal profiles containing AT1 receptors. The gp91phox-labeled dendrites received inputs from large axon terminals resembling vagal afferents. In parallel experiments using patch clamp of dissociated NTS neurons anterogradely labeled via the vagus, we found that AngII potentiates the L-type Ca2+ currents, an effect mediated by AT1 receptors and abolished by the ROS scavenger Mn(III) tetrakis (4-benzoic acid) porphyrin chloride. The NADPH oxidase assembly inhibitor apocynin and the peptide inhibitor gp91phox docking sequence, but not its scrambled version, also blocked the potentiation. The results provide evidence that NADPH oxidase-derived ROS are involved in the effects of AngII on Ca2+ influx in NTS neurons receiving vagal afferents and support the notion that ROS are important signaling molecules in central autonomic networks.
Figures



Similar articles
-
Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius.Hypertension. 2006 Sep;48(3):482-9. doi: 10.1161/01.HYP.0000236647.55200.07. Epub 2006 Aug 7. Hypertension. 2006. PMID: 16894058
-
Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals.Circ Res. 2004 Nov 12;95(10):1019-26. doi: 10.1161/01.RES.0000148637.85595.c5. Epub 2004 Oct 21. Circ Res. 2004. PMID: 15499027
-
Changes in the subcellular distribution of NADPH oxidase subunit p47phox in dendrites of rat dorsomedial nucleus tractus solitarius neurons in response to chronic administration of hypertensive agents.Exp Neurol. 2007 Jun;205(2):383-95. doi: 10.1016/j.expneurol.2007.02.016. Epub 2007 Mar 3. Exp Neurol. 2007. PMID: 17418121 Free PMC article.
-
Angiotensin peptides as neurotransmitters/neuromodulators in the dorsomedial medulla.Clin Exp Pharmacol Physiol. 2002 May-Jun;29(5-6):473-82. doi: 10.1046/j.1440-1681.2002.03659.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12010195 Review.
-
Redox control of cardiovascular homeostasis by angiotensin II.Curr Pharm Des. 2013;19(17):3022-32. doi: 10.2174/1381612811319170008. Curr Pharm Des. 2013. PMID: 23176215 Review.
Cited by
-
Aging, Angiotensin system and dopaminergic degeneration in the substantia nigra.Aging Dis. 2011 Jun;2(3):257-74. Epub 2011 Apr 20. Aging Dis. 2011. PMID: 22396877 Free PMC article.
-
Differences in oxidative stress status and expression of MKP-1 in dorsal medulla of transgenic rats with altered brain renin-angiotensin system.Am J Physiol Regul Integr Comp Physiol. 2012 Oct 15;303(8):R799-806. doi: 10.1152/ajpregu.00566.2011. Epub 2012 Aug 22. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22914751 Free PMC article.
-
Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus.Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2032-42. doi: 10.1152/ajpheart.00568.2008. Epub 2008 Sep 12. Am J Physiol Heart Circ Physiol. 2008. PMID: 18790834 Free PMC article.
-
Central command dysfunction in rats with heart failure is mediated by brain oxidative stress and normalized by exercise training.J Physiol. 2014 Sep 1;592(17):3917-31. doi: 10.1113/jphysiol.2014.272377. Epub 2014 Jun 27. J Physiol. 2014. PMID: 24973409 Free PMC article.
-
PVN Blockade of p44/42 MAPK Pathway Attenuates Salt-induced Hypertension through Modulating Neurotransmitters and Attenuating Oxidative Stress.Sci Rep. 2017 Feb 22;7:43038. doi: 10.1038/srep43038. Sci Rep. 2017. PMID: 28225041 Free PMC article.
References
-
- Ahem GP, Klyachko VA, Jackson MB (2002) cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25: 510-517. - PubMed
-
- Aicher SA, Goldberg A, Sharma S, Pickel VM (2000) μ-Opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. J Comp Neurol 422: 181-190. - PubMed
-
- Andresen MC, Mendelowitz D (1996) Sensory afferent neurotransmission in caudal nucleus tractus solitarius-common denominators. Chem Senses 21: 387-395. - PubMed
-
- Annunziato L, Pannaccione A, Cataldi M, Secondo A, Castaldo P, Di Renzo G, Taglialatela M (2002) Modulation of ion channels by reactive oxygen and nitrogen species: a pathophysiological role in brain aging? Neurobiol Aging 23: 819-834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
