Synaptic contributions to focal and widespread spatiotemporal dynamics in the isolated rat subiculum in vitro
- PMID: 15201325
- PMCID: PMC6729319
- DOI: 10.1523/JNEUROSCI.0309-04.2004
Synaptic contributions to focal and widespread spatiotemporal dynamics in the isolated rat subiculum in vitro
Abstract
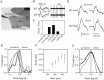
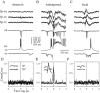
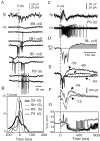
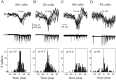
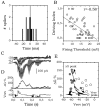
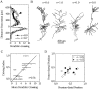
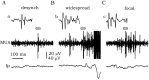
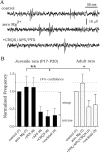
The subiculum, which has a strategic position in controlling hippocampal activity, is receiving significant attention in epilepsy research. However, the functional organization of subicular circuits remains unknown. Here, we combined different recording and analytical methods to study focal and widespread population activity in the isolated subiculum in zero Mg2+ media. Patch and field recordings were combined to examine the contribution of different cell types to population activity. The properties of cells leading field activity were examined. Predictive factors for a cell to behave as leader included exhibiting the bursting phenotype, displaying a low firing threshold, and having more distal apical dendrites. A subset of bursting cells constituted the first glutamatergic type that led a recruitment process that subsequently activated additional excitatory as well as inhibitory cells. This defined a sequence of synaptic excitation and inhibition that was studied by measuring the associated conductance changes and the evolution of the composite reversal potential. It is shown that inhibition was time-locked to excitation, which shunted excitatory inputs and suppressed firing during focal activity. This was recorded extracellularly as a multi-unit ensemble of active cells, the spatial boundaries of which were controlled by inhibition in contrast to widespread epileptiform activity. Focal activity was not dependent on the preparation or the developmental state because it was also recorded under 5 mm [K+]o and in adult tissue. Our data indicate that the subicular networks can be spontaneously organized as leader-follower local circuits in which excitation is mainly driven by a subset of bursting cells and inhibition controls spatiotemporal firing.
Figures
References
-
- Anderson JS, Carandini M, Ferster D (2000) Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol 84: 909-926. - PubMed
-
- Behr J, Heinemann U (1996) Low Mg2+ induced epileptiform activity in the subiculum before and after disconnection from rat hippocamapl and entorhinal cortex slices. Neurosci Lett 205: 25-28. - PubMed
-
- Behr J, Gloveli T, Heinemann U (1998) The perforant path projection from the medial entorhinal cortex layer III to the subiculum in the rat combined hippocampal-entorhinal cortex slice. Eur J Neurosci 10: 1011-1018. - PubMed
-
- Berger TW, Swanson GW, Milner TA, Lynch GS, Thompson RF (1980) Reciprocal anatomical connections between hippocampus and subiculum in the rabbit evidence for subicular innervation of regio superior. Brain Res 183: 265-276. - PubMed
-
- Borg-Graham LJ (2001) The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nat Neurosci 4: 176-183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
