Uropathic observations in mice expressing a constitutively active point mutation in the 5-HT3A receptor subunit
- PMID: 15201326
- PMCID: PMC6729324
- DOI: 10.1523/JNEUROSCI.5658-03.2004
Uropathic observations in mice expressing a constitutively active point mutation in the 5-HT3A receptor subunit
Abstract
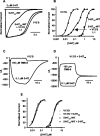
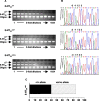
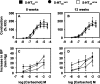
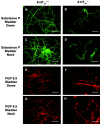
Mutant mice with a hypersensitive serotonin (5-HT)3A receptor were generated through targeted exon replacement. A valine to serine mutation (V13'S) in the channel-lining M2 domain of the 5-HT3A receptor subunit rendered the 5-HT3 receptor 70-fold more sensitive to serotonin and produced constitutive activity when combined with the 5-HT3B subunit. Mice homozygous for the mutant allele (5-HT3Avs/vs) had decreased levels of 5-HT3A mRNA. Measurements on sympathetic ganglion cells in these mice showed that whole-cell serotonin responses were reduced, and that the remaining 5-HT3 receptors were hypersensitive. Male 5-HT3Avs/vs mice died at 2-3 months of age, and heterozygous (5-HT3Avs/+) males and homozygous mutant females died at 4-6 months of age from an obstructive uropathy. Both male and female 5-HT3A mutant mice had urinary bladder mucosal and smooth muscle hyperplasia and hypertrophy, whereas male mutant mice had additional prostatic smooth muscle and urethral hyperplasia. 5-HT3A mutant mice had marked voiding dysfunction characterized by a loss of micturition contractions with overflow incontinence. Detrusor strips from 5-HT3Avs/vs mice failed to contract to neurogenic stimulation, despite overall normal responses to a cholinergic agonist, suggestive of altered neuronal signaling in mutant mouse bladders. Consistent with this hypothesis, decreased nerve fiber immunoreactivity was observed in the urinary bladders of 5-HT3Avs/vs compared with 5-HT3A wild-type (5-HT3A+/+) mice. These data suggest that persistent activation of the hypersensitive and constitutively active 5-HT3A receptor in vivo may lead to excitotoxic neuronal cell death and functional changes in the urinary bladder, resulting in bladder hyperdistension, urinary retention, and overflow incontinence.
Figures

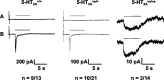



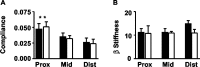
Similar articles
-
A single channel mutation alters agonist efficacy at 5-HT3A and 5-HT3AB receptors.Br J Pharmacol. 2013 Sep;170(2):391-402. doi: 10.1111/bph.12287. Br J Pharmacol. 2013. PMID: 23822584 Free PMC article.
-
The 4'lysine in the putative channel lining domain affects desensitization but not the single-channel conductance of recombinant homomeric 5-HT3A receptors.J Physiol. 2000 Jan 15;522 Pt 2(Pt 2):187-98. doi: 10.1111/j.1469-7793.2000.00187.x. J Physiol. 2000. PMID: 10639097 Free PMC article.
-
Importance of recognition loops B and D in the activation of human 5-HT₃ receptors by 5-HT and meta-chlorophenylbiguanide.Neuropharmacology. 2013 Oct;73:398-403. doi: 10.1016/j.neuropharm.2013.06.017. Epub 2013 Jun 26. Neuropharmacology. 2013. PMID: 23810831
-
The 5-hydroxytryptamine type 3 (5-HT3) receptor reveals a novel determinant of single-channel conductance.Biochem Soc Trans. 2004 Jun;32(Pt3):547-52. doi: 10.1042/BST0320547. Biochem Soc Trans. 2004. PMID: 15157181 Review.
-
Alterations in the physiological properties of urinary bladder smooth muscle caused by bladder emptying against an obstruction.Scand J Urol Nephrol Suppl. 1997;184:51-8. Scand J Urol Nephrol Suppl. 1997. PMID: 9165623 Review. No abstract available.
Cited by
-
Auto-inhibition at a ligand-gated ion channel: a cross-talk between orthosteric and allosteric sites.Br J Pharmacol. 2015 Jan;172(1):93-105. doi: 10.1111/bph.12896. Epub 2014 Nov 24. Br J Pharmacol. 2015. PMID: 25176133 Free PMC article.
-
The 5-HT3 receptor as a therapeutic target.Expert Opin Ther Targets. 2007 Apr;11(4):527-40. doi: 10.1517/14728222.11.4.527. Expert Opin Ther Targets. 2007. PMID: 17373882 Free PMC article. Review.
-
Dynamic Expression of Serotonin Receptor 5-HT3A in Developing Sensory Innervation of the Lower Urinary Tract.Front Neurosci. 2017 Jan 6;10:592. doi: 10.3389/fnins.2016.00592. eCollection 2016. Front Neurosci. 2017. PMID: 28111539 Free PMC article.
-
Hydroxy Pentacyclic Triterpene Acid, Kaempferol, Inhibits the Human 5-Hydroxytryptamine Type 3A Receptor Activity.Int J Mol Sci. 2022 Jan 4;23(1):544. doi: 10.3390/ijms23010544. Int J Mol Sci. 2022. PMID: 35008969 Free PMC article.
-
5-HT3 Signaling Alters Development of Sacral Neural Crest Derivatives That Innervate the Lower Urinary Tract.Int J Mol Sci. 2021 Jun 25;22(13):6838. doi: 10.3390/ijms22136838. Int J Mol Sci. 2021. PMID: 34202161 Free PMC article.
References
-
- Barras M, Van der Graaf PH, Angel I (1996) Characterization of the 5-HT receptor potentiating neurotransmission in rabbit bladder. Eur J Pharmacol 318: 425-428. - PubMed
-
- Bassuk JA, Grady R, Mitchell M (2000) The molecular era of bladder research: transgenic mice as experimental tools in the study of outlet obstruction. J Urol 164: 170-179. - PubMed
-
- Broide RS, Salas R, Ji D, Paylor R, Patrick JW, Dani JA, De Biasi M (2002) Increased sensitivity to nicotine-induced seizures in mice expressing the L250T α7 nicotinic acetylcholine receptor mutation. Mol Pharmacol 61: 695-705. - PubMed
-
- Charlton RG, Morley AR, Chambers P, Gillespie JI (1999) Focal changes in nerve, muscle and connective tissue in normal and unstable human bladder. BJU International 84: 953-960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
