Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord
- PMID: 15201327
- PMCID: PMC6729333
- DOI: 10.1523/JNEUROSCI.2719-03.2004
Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord
Abstract
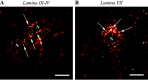
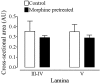
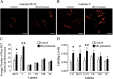
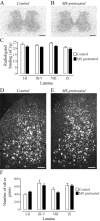
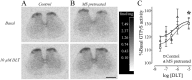
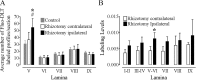
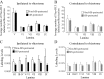
An in vivo fluorescent deltorphin (Fluo-DLT) internalization assay was used to assess the distribution and regulation of pharmacologically available delta opioid receptors (deltaORs) in the rat lumbar (L4-5) spinal cord. Under basal conditions, intrathecal injection of Fluo-DLT resulted in the labeling of numerous deltaOR-internalizing neurons throughout dorsal and ventral horns. The distribution and number of Fluo-DLT-labeled perikaryal profiles were consistent with that of deltaOR-expressing neurons, as revealed by in situ hybridization and immunohistochemistry, suggesting that a large proportion of these cells was responsive to intrathecally administered deltaOR agonists. Pretreatment of rats with morphine for 48 hr resulted in a selective increase in Fluo-DLT-labeled perikaryal profiles within the dorsal horn. These changes were not accompanied by corresponding augmentations in either deltaOR mRNA or (125)I-deltorphin-II binding levels, suggesting that they were attributable to higher densities of cell surface deltaOR available for internalization rather than to enhanced production of the receptor. Unilateral dorsal rhizotomy also resulted in increased Fluo-DLT internalization in the ipsilateral dorsal horn when compared with the side contralateral to the deafferentation or to non-deafferented controls, suggesting that deltaOR trafficking in dorsal horn neurons may be regulated by afferent inputs. Furthermore, morphine treatment no longer increased Fluo-DLT internalization on either side of the spinal cord after unilateral dorsal rhizotomy, indicating that microOR-induced changes in the cell surface availability of deltaOR depend on the integrity of primary afferent inputs. Together, these results suggest that regulation of deltaOR responsiveness through microOR activation in this region is linked to somatosensory information processing.
Figures



Similar articles
-
Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice.J Neurosci. 2003 Jun 15;23(12):4888-98. doi: 10.1523/JNEUROSCI.23-12-04888.2003. J Neurosci. 2003. PMID: 12832511 Free PMC article.
-
Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception.J Neurosci. 2001 Oct 1;21(19):7598-607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. J Neurosci. 2001. PMID: 11567050 Free PMC article.
-
Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin.Neuroscience. 2007 Jan 5;144(1):263-74. doi: 10.1016/j.neuroscience.2006.08.077. Epub 2006 Oct 19. Neuroscience. 2007. PMID: 17055663
-
Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia.J Neurosci. 2006 Jan 18;26(3):953-62. doi: 10.1523/JNEUROSCI.3598-05.2006. J Neurosci. 2006. PMID: 16421315 Free PMC article.
-
Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain.Pain. 2004 Jun;109(3):266-273. doi: 10.1016/j.pain.2004.01.011. Pain. 2004. PMID: 15157687
Cited by
-
Implication of delta opioid receptor subtype 2 but not delta opioid receptor subtype 1 in the development of morphine analgesic tolerance in a rat model of chronic inflammatory pain.Eur J Neurosci. 2015 Apr;41(7):901--7. doi: 10.1111/ejn.12829. Epub 2015 Jan 9. Eur J Neurosci. 2015. PMID: 25639561 Free PMC article.
-
Regulation of μ and δ opioid receptor functions: involvement of cyclin-dependent kinase 5.Br J Pharmacol. 2015 May;172(10):2573-87. doi: 10.1111/bph.13088. Epub 2015 Mar 24. Br J Pharmacol. 2015. PMID: 25598508 Free PMC article.
-
Recent advances on the δ opioid receptor: from trafficking to function.Br J Pharmacol. 2015 Jan;172(2):403-19. doi: 10.1111/bph.12706. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24665909 Free PMC article. Review.
-
Targeting G protein-coupled receptor for pain management.Brain Circ. 2017 Apr-Jun;3(2):109-113. doi: 10.4103/bc.bc_3_17. Epub 2017 Jul 18. Brain Circ. 2017. PMID: 30276310 Free PMC article. Review.
-
Tryptophan Substitution in CJ-15,208 (cyclo[Phe-D-Pro-Phe-Trp]) Introduces δ-Opioid Receptor Antagonism, Preventing Antinociceptive Tolerance and Stress-Induced Reinstatement of Extinguished Cocaine-Conditioned Place Preference.Pharmaceuticals (Basel). 2023 Aug 29;16(9):1218. doi: 10.3390/ph16091218. Pharmaceuticals (Basel). 2023. PMID: 37765026 Free PMC article.
References
-
- Abbadie C, Pan YX, Pasternak GW (2000) Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol 419: 244-256. - PubMed
-
- Abbadie C, Pasternak GW, Aicher SA (2001) Presynaptic localization of the carboxy-terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience 106: 833-842. - PubMed
-
- Abbadie C, Lombard MC, Besson JM, Trafton JA, Basbaum AI (2002) Mu and delta opioid receptor-like immunoreactivity in the cervical spinal cord of the rat after dorsal rhizotomy or neonatal capsaicin: an analysis of pre- and postsynaptic receptor distributions. Brain Res 930: 150-162. - PubMed
-
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hokfelt T, Zhou Z, Zhang X (2003) Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron 37: 121-133. - PubMed
