Paradoxical potentiation of neuronal T-type Ca2+ current by ATP at resting membrane potential
- PMID: 15201332
- PMCID: PMC6729316
- DOI: 10.1523/JNEUROSCI.1038-04.2004
Paradoxical potentiation of neuronal T-type Ca2+ current by ATP at resting membrane potential
Abstract
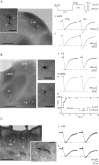
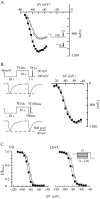
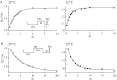
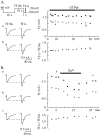
Despite the marked influence on neuronal physiology of the low-voltage activated T-type Ca(2+) currents, little is known about the intracellular pathways and neurotransmitters involved in their regulations. Here, we report that in thalamocortical neurons a phosphorylation mechanism induces an increase both in the current amplitude (1.5 +/- 0.27-fold in the ventrobasal nucleus) and its inactivation kinetics. Dialysis of the neuron with an ATP-free solution suppresses the T-current potentiation, whereas it becomes irreversible in the presence of ATPgammaS. Phosphorylation occurs when the channels are inactivated and is slowly removed when they recover from inactivation and remain in closed states (time constants of the induction and removal of the potentiation: 579 +/- 143 msec and 4.9 +/- 1.1 sec, respectively, at 25 degrees C). The resulting apparent voltage sensitivity of this regulation follows the voltage dependence of the current steady-state inactivation. Thus, the current is paradoxically inhibited when the preceding hyperpolarization is lengthened, and maximal currents are generated after transient hyperpolarizations with a duration (0.7-1.5 sec) that is defined by the balance between the kinetics of the dephosphorylation and deinactivation. In addition, the phosphorylation will facilitate the generation of T current at resting membrane potential. This potentiation, which is specific to sensory thalamocortical neurons, would markedly influence the electroresponsiveness of these neurons and represent the first evidence of a regulation of native Cav3.1 channels.
Figures





Similar articles
-
Recovery from inactivation of t-type ca2+ channels in rat thalamic neurons.J Neurosci. 2001 Mar 15;21(6):1884-92. doi: 10.1523/JNEUROSCI.21-06-01884.2001. J Neurosci. 2001. PMID: 11245673 Free PMC article.
-
Cytosolic ATP Relieves Voltage-Dependent Inactivation of T-Type Calcium Channels and Facilitates Excitability of Neurons in the Rat Central Medial Thalamus.eNeuro. 2018 Feb 15;5(1):ENEURO.0016-18.2018. doi: 10.1523/ENEURO.0016-18.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29468189 Free PMC article.
-
Voltage-dependent potentiation of low-voltage-activated Ca2+ channel currents in cultured rat bone marrow cells.J Physiol. 1995 Dec 15;489 ( Pt 3)(Pt 3):649-61. doi: 10.1113/jphysiol.1995.sp021080. J Physiol. 1995. PMID: 8788931 Free PMC article.
-
Modulation of neuronal T-type calcium channels.CNS Neurol Disord Drug Targets. 2006 Dec;5(6):611-27. doi: 10.2174/187152706779025544. CNS Neurol Disord Drug Targets. 2006. PMID: 17168746 Review.
-
Density is destiny--on [corrected] the relation between quantity of T-type Ca2+ channels and neuronal electrical behavior.CNS Neurol Disord Drug Targets. 2006 Dec;5(6):655-62. doi: 10.2174/187152706779025517. CNS Neurol Disord Drug Targets. 2006. PMID: 17168749 Review.
Cited by
-
Developmentally Regulated Rebound Depolarization Enhances Spike Timing Precision in Auditory Midbrain Neurons.Front Cell Neurosci. 2020 Aug 6;14:236. doi: 10.3389/fncel.2020.00236. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32848625 Free PMC article.
-
T-type calcium channels consolidate tonic action potential output of thalamic neurons to neocortex.J Neurosci. 2012 Aug 29;32(35):12228-36. doi: 10.1523/JNEUROSCI.1362-12.2012. J Neurosci. 2012. PMID: 22933804 Free PMC article.
-
Midline thalamic paraventricular nucleus neurons display diurnal variation in resting membrane potentials, conductances, and firing patterns in vitro.J Neurophysiol. 2012 Apr;107(7):1835-44. doi: 10.1152/jn.00974.2011. Epub 2012 Jan 4. J Neurophysiol. 2012. PMID: 22219029 Free PMC article.
-
Modeling thalamocortical cell: impact of ca channel distribution and cell geometry on firing pattern.Front Comput Neurosci. 2008 Dec 12;2:5. doi: 10.3389/neuro.10.005.2008. eCollection 2008. Front Comput Neurosci. 2008. PMID: 19129908 Free PMC article.
-
T-type Ca2+ channels in absence epilepsy.Pflugers Arch. 2014 Apr;466(4):719-34. doi: 10.1007/s00424-014-1461-y. Epub 2014 Feb 13. Pflugers Arch. 2014. PMID: 24519464 Review.
References
-
- Alvarez JL, Artiles A, Talavera K, Vassort G (2000) Modulation of voltage-dependent facilitation of the T-type calcium current by sodium ion in isolated frog atrial cells. Pflügers Arch 441: 39-48. - PubMed
-
- Arnoult C, Villaz M, Florman HM (1998) Pharmacological properties of the T-type Ca2+ current of mouse spermatogenic cells. Mol Pharmacol 53: 1104-1111. - PubMed
-
- Artalejo CR, Rossie S, Perlman RL, Fox AP (1992) Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature 358: 63-66. - PubMed
-
- Bean BP (1989) Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153-156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
