Incomplete glycosylation and defective intracellular targeting of mutant solute carrier family 11 member 1 (Slc11a1)
- PMID: 15202932
- PMCID: PMC1133956
- DOI: 10.1042/BJ20040808
Incomplete glycosylation and defective intracellular targeting of mutant solute carrier family 11 member 1 (Slc11a1)
Abstract
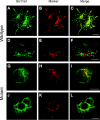
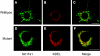
Solute carrier family 11 member 1 (Slc11a1, formerly Nramp1) is a highly glycosylated, 12 transmembrane domain protein expressed in macrophages. It resides in the membrane of late endosomes and lysosomes, where it functions as a bivalent cation transporter. Mice susceptible to infection by various intracellular pathogens including Leishmania donovani and Salmonella typhimurium carry a glycine to aspartic acid substitution at position 169 (G169D, Gly(169)-->Asp), within transmembrane domain 4 of Slc11a1. To investigate the molecular pathogenesis of infectious disease susceptibility, we compared the behaviour of heterologously and endogenously expressed wild-type and mutant Slc11a1 by immunofluorescence, immunoelectron microscopy and Western-blot analysis. We found occasional late endosome/lysosome staining of mutant protein using immunoelectron microscopy, but most of the mutant Slc11a1 was retained within the ER (endoplasmic reticulum). Using glycosylation as a marker for protein maturation in two independent heterologous expression systems, we found that most mutant Slc11a1 existed as an ER-dependent, partially glycosylated intermediate species. Correct endosomal targeting of wild-type Slc11a1 continued despite disruption of N-glycosylation sites, indicating that glycosylation did not influence folding or sorting. We propose that the G169D mutation causes localized misfolding of Slc11a1, resulting in its retention in the ER and manifestation of the loss of function phenotype.
Figures





Similar articles
-
Solute carrier 11a1 (Slc11a1; formerly Nramp1) regulates metabolism and release of iron acquired by phagocytic, but not transferrin-receptor-mediated, iron uptake.Biochem J. 2002 Apr 1;363(Pt 1):89-94. doi: 10.1042/0264-6021:3630089. Biochem J. 2002. PMID: 11903051 Free PMC article.
-
Structure and topology of Slc11a1(164-191) with G169D mutation in membrane-mimetic environments.J Struct Biol. 2009 Jan;165(1):27-36. doi: 10.1016/j.jsb.2008.09.008. Epub 2008 Oct 4. J Struct Biol. 2009. PMID: 18929666
-
Slc11a1, formerly Nramp1, is expressed in dendritic cells and influences major histocompatibility complex class II expression and antigen-presenting cell function.Infect Immun. 2007 Oct;75(10):5059-67. doi: 10.1128/IAI.00153-07. Epub 2007 Jul 9. Infect Immun. 2007. PMID: 17620357 Free PMC article.
-
Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story.Immunol Lett. 2003 Jan 22;85(2):197-203. doi: 10.1016/s0165-2478(02)00231-6. Immunol Lett. 2003. PMID: 12527228 Review.
-
SLC11A1 (formerly NRAMP1) and disease resistance.Cell Microbiol. 2001 Dec;3(12):773-84. doi: 10.1046/j.1462-5822.2001.00150.x. Cell Microbiol. 2001. PMID: 11736990 Free PMC article. Review. No abstract available.
Cited by
-
Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival.Immunity. 2013 Oct 17;39(4):697-710. doi: 10.1016/j.immuni.2013.09.006. Immunity. 2013. PMID: 24138881 Free PMC article.
-
The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome.J Leukoc Biol. 2008 Sep;84(3):689-700. doi: 10.1189/jlb.1107781. Epub 2008 Jun 27. J Leukoc Biol. 2008. PMID: 18586980 Free PMC article.
-
Structural characterization and molecular dynamics simulations of the caprine and bovine solute carrier family 11 A1 (SLC11A1).J Comput Aided Mol Des. 2019 Feb;33(2):265-285. doi: 10.1007/s10822-018-0179-x. Epub 2018 Dec 12. J Comput Aided Mol Des. 2019. PMID: 30543052
-
An immunoaffinity-based method for isolating ultrapure adult astrocytes based on ATP1B2 targeting by the ACSA-2 antibody.J Biol Chem. 2017 May 26;292(21):8874-8891. doi: 10.1074/jbc.M116.765313. Epub 2017 Apr 3. J Biol Chem. 2017. PMID: 28373281 Free PMC article.
-
Iron in intracellular infection: to provide or to deprive?Front Cell Infect Microbiol. 2013 Dec 9;3:96. doi: 10.3389/fcimb.2013.00096. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24367768 Free PMC article. Review.
References
-
- Blackwell J. M., Searle S., Mohamed H., White J. K. Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol. Lett. 2003;85:197–203. - PubMed
-
- Cellier M., Shustik C., Dalton W., Rich E., Hu J., Malo D., Schurr E., Gros P. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J. Leukoc. Biol. 1997;61:96–105. - PubMed
-
- Clifford K. S., MacDonald M. J. Survey of mRNAs encoding zinc transporters and other metal complexing proteins in pancreatic islets of rats from birth to adulthood: similar patterns in the Sprague–Dawley and Wistar BB strains. Diabetes Res. Clin. Pract. 2000;49:77–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

