Identification of two novel alternatively spliced Neuropilin-1 isoforms
- PMID: 15203206
- PMCID: PMC2868064
- DOI: 10.1016/j.ygeno.2004.02.001
Identification of two novel alternatively spliced Neuropilin-1 isoforms
Abstract
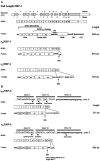
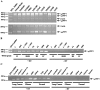
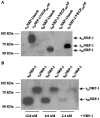
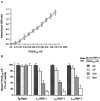
Neuropilin-1 (NRP1) is a coreceptor to a tyrosine kinase receptor for both the vascular endothelial growth factor (VEGF) family and semaphorin (Sema) family members. NRP1 plays versatile roles in angiogenesis, axon guidance, cell survival, migration, and invasion. NRP1 contains three distinct extracellular domains, a1a2, b1b2, and c. We report here the identification of two novel soluble human NRP1 isoforms, which we named sIIINRP1 and sIVNRP1. These soluble NRP1 isoforms were generated by alternative splicing of the NRP1 gene, a common regulatory mechanism occurring in cell surface receptor families. Both sIIINRP1 and sIVNRP1 contain a1a2 and b1b2 domains, but no c domain, and the rest of the NRP1 sequence. Additionally, sIIINRP1 is missing 48 amino acids within the C-terminus of the b2 domain. Both sIIINRP1 and sIVNRP1 are expressed in human cancerous and normal tissues. These molecules are capable of binding to VEGF165 and Sema3A. Furthermore, recombinant sIIINRP1 and sIVNRP1 proteins inhibit NRP1-mediated MDA-MB-231 breast cancer cell migration. These results indicate the multiple levels of regulation in NRP1 function and suggest that these two novel NRP1 isoforms are useful antagonists for NRP1-mediated cellular activities.
Copyright 2004 Elsevier Inc.
Figures

Similar articles
-
Characterization of a new alternatively spliced neuropilin-1 isoform.Angiogenesis. 2003;6(1):39-45. doi: 10.1023/a:1025884628155. Angiogenesis. 2003. PMID: 14517403
-
Long isoform of VEGF stimulates cell migration of breast cancer by filopodia formation via NRP1/ARHGAP17/Cdc42 regulatory network.Int J Cancer. 2018 Dec 1;143(11):2905-2918. doi: 10.1002/ijc.31645. Epub 2018 Oct 9. Int J Cancer. 2018. PMID: 29971782 Free PMC article.
-
Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain.J Biol Chem. 2002 Jul 5;277(27):24818-25. doi: 10.1074/jbc.M200730200. Epub 2002 May 1. J Biol Chem. 2002. PMID: 11986311
-
Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis.Angiogenesis. 2008;11(1):31-9. doi: 10.1007/s10456-008-9097-1. Epub 2008 Feb 19. Angiogenesis. 2008. PMID: 18283547 Review.
-
The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF.Adv Exp Med Biol. 2002;515:81-90. doi: 10.1007/978-1-4615-0119-0_7. Adv Exp Med Biol. 2002. PMID: 12613545 Review.
Cited by
-
The Role of Proteoglycans in Cancer Metastasis and Circulating Tumor Cell Analysis.Front Cell Dev Biol. 2020 Aug 26;8:749. doi: 10.3389/fcell.2020.00749. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32984308 Free PMC article. Review.
-
Identification of circulating neuropilin-1 and dose-dependent elevation following anti-neuropilin-1 antibody administration.MAbs. 2009 Jul-Aug;1(4):364-9. doi: 10.4161/mabs.1.4.8885. Epub 2009 Jul 29. MAbs. 2009. PMID: 20068394 Free PMC article.
-
Soluble Neuropilin-1 Is Elevated in Sepsis and Correlates with Organ Dysfunction and Long-Term Mortality in Critical Illness.Int J Mol Sci. 2024 May 16;25(10):5438. doi: 10.3390/ijms25105438. Int J Mol Sci. 2024. PMID: 38791476 Free PMC article.
-
Neuropilin-1 promotes Hedgehog signaling through a novel cytoplasmic motif.J Biol Chem. 2017 Sep 15;292(37):15192-15204. doi: 10.1074/jbc.M117.783845. Epub 2017 Jun 30. J Biol Chem. 2017. PMID: 28667171 Free PMC article.
-
Population pharmacokinetic and pharmacodynamic modeling of MNRP1685A in cynomolgus monkeys using two-target quasi-steady-state (QSS) model.J Pharmacokinet Pharmacodyn. 2012 Apr;39(2):217-26. doi: 10.1007/s10928-012-9244-6. Epub 2012 Mar 1. J Pharmacokinet Pharmacodyn. 2012. PMID: 22382554
References
-
- Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 2002;12(1):13–19. - PubMed
-
- Puschel AW. The function of neuropilin/plexin complexes. Adv. Exp. Med. Biol. 2002;515:71–80. - PubMed
-
- Takagi S, Tsuji T, Amagai T, Takamatsu T, Fujisawa H. Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev. Biol. 1987;122(1):90–100. - PubMed
-
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90(4):753–762. - PubMed
-
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

