White matter lesions in panencephalopathic type of Creutzfeldt-Jakob disease: MR imaging and pathologic correlations
- PMID: 15205123
- PMCID: PMC7975660
White matter lesions in panencephalopathic type of Creutzfeldt-Jakob disease: MR imaging and pathologic correlations
Abstract
Background and purpose: A panencephalopathic type of Creutzfeldt-Jakob disease (pCJD) is characterized by the extensive involvement of the cerebral white matter as well as the cerebral gray matter. It has been a point of controversy, however, whether the white matter changes represent primary or secondary degeneration. The aim of this study was to elucidate, by using MR images and histologic examinations, whether the white matter lesions in pCJD are primary or secondary degeneration.
Methods: Serial changes of T2 hyperintensities and histologic findings of six autopsy-proved cases of pCJD were retrospectively analyzed.
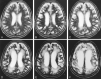
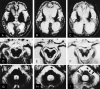
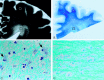
Results: Serial MR images of brains affected by pCJD revealed that T2 hyperintensities appeared in the cerebral gray matter 2-5 months after onset and in the cerebral white matter around the lateral ventricles approximately 5 months after onset. They rapidly extended to deep and subcortical white matter during the next several months and then to the entire cerebral white matter 10 months after onset. Histologic examination of the white matter lesions revealed spongy changes or tissue rarefaction associated with gemistocytic astrocytosis, which indicates primary involvement of the white matter. At the terminal stages of cases with a longer clinical course, MR images showed T2 hyperintensities in the corticospinal tracts in the internal capsule and brain stem, which histologically disclosed loss of myelin and axons accompanied by fibrillary gliosis that indicates secondary degeneration.
Conclusion: Cerebral white matter lesions in pCJD were considered to be primary changes of the disease, but the lesions of the corticospinal tracts were secondary to cortical or cerebral or both white matter lesions.
Figures



Comment in
-
Primary white matter involvement in sporadic-type Creutzfeldt-Jakob disease? Which came first, the chicken or the egg?AJNR Am J Neuroradiol. 2004 Jun-Jul;25(6):905-6. AJNR Am J Neuroradiol. 2004. PMID: 15205120 Free PMC article. No abstract available.
References
-
- Gonatas NK, Terry RD, Weiss M. Electron microscopic study in two cases of Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol 1965;24:575–598 - PubMed
-
- Park TS, Kleinman GM, Richardson EP. Creutzfeldt-Jakob disease with extensive degeneration of white matter. Acta Neuropathol 1980;52:239–242 - PubMed
-
- Monreal J, Collins GH, Masters CL, et al. Creutzfeldt-Jakob disease in an adolescent. J Neurol Sci 1981;52:341–350 - PubMed
-
- Macchi G, Abbamondi AL, Di Trapani G, et al. On white matter lesions of the Creutzfeldt-Jakob disease. J Neurol Sci 1984;63:197–206 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
