A comparison of the fixed combination of latanoprost and timolol with the unfixed combination of brimonidine and timolol in patients with elevated intraocular pressure. A six month, evaluator masked, multicentre study in Europe
- PMID: 15205229
- PMCID: PMC1772215
- DOI: 10.1136/bjo.2003.029330
A comparison of the fixed combination of latanoprost and timolol with the unfixed combination of brimonidine and timolol in patients with elevated intraocular pressure. A six month, evaluator masked, multicentre study in Europe
Abstract
Purpose: To compare the intraocular pressure (IOP) reducing effect and safety of fixed combination (FC) latanoprost/timolol with unfixed combination (UFC) brimonidine/timolol in patients with increased IOP.
Methods: In this 6 month, randomised, evaluator masked, parallel group European study, patients with glaucoma or ocular hypertension and IOP > or =21 mm Hg on monotherapy or >16 mm Hg on dual therapy received either FC latanoprost/timolol at 8:00AM or UFC brimonidine/timolol at 8:00AM and 8:00PM. The primary outcome was the difference from baseline to month 6 in mean diurnal IOP reduction.
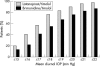
Results: 325 of 334 randomised patients were included in intent to treat analyses (FC latanoprost/timolol, 163; UFC brimonidine/timolol, 162). Baseline diurnal IOP levels were similar: FC latanoprost/timolol, 26.4 (SD 2.7) mm Hg; UFC brimonidine/timolol, 26.5 (SD 2.8) mm Hg (p = 0.851). At month 6, levels were 16.9 (SD 2.8) mm Hg in FC latanoprost/timolol patients and 18.2 (SD 3.1) mm Hg in UFC brimonidine/timolol patients (p<0.001). No adverse events were reported by 76.4% and 75.5% of patients receiving FC latanoprost/timolol versus UFC brimonidine/timolol, respectively. Larger proportions of brimonidine/timolol treated patients reported study medication related adverse events (18.6% v 7.3%) and discontinued study participation because of this (10.8% v 1.8%).
Conclusion: Fixed combination latanoprost/timolol administered once daily is both more effective and better tolerated than twice daily dosing with UFC brimonidine/timolol.
Figures

References
-
- American Academy of Ophthalmology. Preferred practice pattern: primary open-angle glaucoma. San Francisco, CA: American Academy of Ophthalmology, 2000.
-
- European Glaucoma Society Committee for Education and Research. Terminology and Guidelines for Glaucoma. Savona, Italy: DOGMA S.r.l., 1998.
-
- O’Connor DJ, Martone JF, Mead AM. Additive intraocular pressure lowering effect of various medications with latanoprost. Am J Ophthalmol 2002;133:836–7. - PubMed
-
- Hartenbaum D . The efficacy of dorzolamide, a topical carbonic anhydrase inhibitor, in combination with timolol in the treatment of patients with open-angle glaucoma and ocular hypertension. Clin Ther 1996;18:460–5. - PubMed
-
- Diestelhorst M , Almegård B. Comparison of two fixed combinations of latanoprost and timolol in open-angle glaucoma. Graefe’s Arch Clin Exp Ophthalmol 1998;236:577–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
