Autogenous temporalis fascia patch graft for porous polyethylene (Medpor) sphere orbital implant exposure
- PMID: 15205243
- PMCID: PMC1772235
- DOI: 10.1136/bjo.2003.026823
Autogenous temporalis fascia patch graft for porous polyethylene (Medpor) sphere orbital implant exposure
Abstract
Background: Temporalis fascia has been recommended for hydroxyapatite sphere exposure. The aim of this study was to identify potential risk factors for exposure of porous polyethylene (Medpor) sphere implants and evaluate the use of autogenous temporalis fascia as a patch graft for exposure.
Methods: A retrospective review of consecutive cases of porous polyethylene sphere orbital implant exposure.
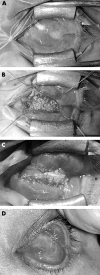
Results: Five cases presented between May 2000 and October 2001 (three males, two females; mean age 44.5 years). Three had enucleation (two with primary implants) and two had evisceration (one with primary implant). Exposure occurred in one primary, two secondary, and two replacement implants. Orbital implant diameter was 20 mm in four cases and 16 mm in one case (contracted socket). The mean time from implantation to exposure was 23 months (range 0.7-42.6). Three patients had secondary motility peg placement before exposure. The average time from last procedure (sphere implant or peg insertion) to exposure was 3 months (range 0.7-12.6). Four patients required surgical intervention, of which three needed more than one procedure. Autogenous temporalis fascia grafting successfully closed the defect without re-exposure in three of these four patients. The grafts were left bare in three patients, with a mean time to conjunctivalise of 2.4 months (range 1.6-3.2).
Conclusions: Exposed porous polyethylene sphere implants were treated successfully with autogenous temporalis fascia graft in three of four patients. This technique is useful, the graft easy to harvest, and did not lead to prolonged socket inflammation, infection, or extrusion.
Figures

Similar articles
-
Amniotic membrane transplantation for porous sphere orbital implant exposure.J Zhejiang Univ Sci B. 2007 Sep;8(9):616-9. doi: 10.1631/jzus.2007.B0616. J Zhejiang Univ Sci B. 2007. PMID: 17726741 Free PMC article.
-
Extrusion of enucleation implants: treatment with secondary implants and autogenous temporalis fascia or fascia lata patch grafts.Ophthalmic Surg. 1992 Jul;23(7):472-6. Ophthalmic Surg. 1992. PMID: 1407945
-
[Porous polyethylene (Medpor) orbital implant. Prospective study of 75 primary implantations].J Fr Ophtalmol. 2001 Dec;24(10):1067-73. J Fr Ophtalmol. 2001. PMID: 11913237 Clinical Trial. French.
-
Porous polyethylene orbital implant in the pediatric population.Am J Ophthalmol. 2004 Sep;138(3):425-9. doi: 10.1016/j.ajo.2004.04.062. Am J Ophthalmol. 2004. PMID: 15364225 Review.
-
Orbital implants insertion to improve ocular prostheses motility.J Craniofac Surg. 2010 May;21(3):870-5. doi: 10.1097/SCS.0b013e3181d80904. J Craniofac Surg. 2010. PMID: 20485072 Review.
Cited by
-
The efficacy of acrylic acid grafting and arginine-glycine-aspartic acid peptide immobilization on fibrovascular ingrowth into porous polyethylene implants in rabbits.Graefes Arch Clin Exp Ophthalmol. 2007 Jun;245(6):855-62. doi: 10.1007/s00417-006-0475-3. Epub 2006 Nov 22. Graefes Arch Clin Exp Ophthalmol. 2007. PMID: 17119998
-
Amniotic membrane transplantation for porous sphere orbital implant exposure.J Zhejiang Univ Sci B. 2007 Sep;8(9):616-9. doi: 10.1631/jzus.2007.B0616. J Zhejiang Univ Sci B. 2007. PMID: 17726741 Free PMC article.
-
Use of extraocular muscle flaps in the correction of orbital implant exposure.PLoS One. 2013 Sep 25;8(9):e72223. doi: 10.1371/journal.pone.0072223. eCollection 2013. PLoS One. 2013. PMID: 24086260 Free PMC article.
-
Effect of basic fibroblast growth factor (bFGF) on the treatment of exposure of the orbital implants.J Zhejiang Univ Sci B. 2007 Sep;8(9):620-5. doi: 10.1631/jzus.2007.B0620. J Zhejiang Univ Sci B. 2007. PMID: 17726742 Free PMC article.
-
Complications of orbital endoimplantation in the Eye Clinic of the Lithuanian University of Health Sciences.Acta Med Litu. 2017;24(2):101-106. doi: 10.6001/actamedica.v24i2.3490. Acta Med Litu. 2017. PMID: 28845127 Free PMC article.
References
-
- Danz W Sr. Mobility implants: a review. Adv Ophthalmic Plast Reconstr Surg 1990;8:46–52. - PubMed
-
- Karesh JW, Dresner SC. High-density porous polyethylene (Medpor) as a successful anophthalmic socket implant. Ophthalmology 1994;101:1688–95, discussion 1695–6. - PubMed
-
- Fan JT, Robertson DM. Long-term follow-up of the Allen implant. 1967 to 1991. Ophthalmology 1995;102:510–16. - PubMed
-
- De Potter P , Duprez T, Cosnard G. Postcontrast magnetic resonance imaging assessment of porous polyethylene orbital implant (Medpor). Ophthalmology 2000;107:1656–60. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
