The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH
- PMID: 15205416
- PMCID: PMC421590
- DOI: 10.1128/JB.186.13.4152-4158.2004
The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH
Abstract
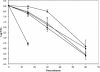
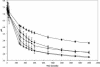
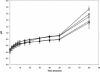
Previously, it has been demonstrated that the membrane fatty acid composition of Streptococcus mutans is affected by growth pH (E. M. Fozo and R. G. Quivey, Jr., Appl. Environ. Microbiol. 70:929-936, 2004; R. G. Quivey, Jr., R. Faustoferri, K. Monahan, and R. Marquis, FEMS Microbiol. Lett. 189:89-92, 2000). Specifically, the proportion of monounsaturated fatty acids increases when the organism is grown in acidic environments; if the shift to increased monounsaturated fatty acids is blocked by the addition of a fatty acid biosynthesis inhibitor, the organism is rendered more acid sensitive (E. M. Fozo and R. G. Quivey, Jr., Appl. Environ. Microbiol. 70:929-936, 2004). Recently, work with Streptococcus pneumoniae has identified a novel enzyme, FabM, responsible for the production of monounsaturated fatty acids (H. Marrakchi, K. H. Choi, and C. O. Rock, J. Biol. Chem. 277:44809-44816, 2002). Using the published S. pneumoniae sequence, a putative FabM was identified in the S. mutans strain UA159. We generated a fabM strain that does not produce unsaturated fatty acids as determined by gas chromatography of fatty acid methyl esters. The mutant strain was extremely sensitive to low pH in comparison to the wild type; however, the acid-sensitive phenotype was relieved by growth in the presence of long-chain monounsaturated fatty acids or through genetic complementation. The strain exhibited reduced glycolytic capability and altered glucose-PTS activity. In addition, the altered membrane composition was more impermeable to protons and did not maintain a normal DeltapH. The results suggest that altered membrane composition can significantly affect the acid survival capabilities, as well as several enzymatic activities, of S. mutans.
Figures

Similar articles
-
Fatty-acid profiles and expression of the fabM gene in a fluoride-resistant strain of Streptococcus mutans.Arch Oral Biol. 2012 Jan;57(1):10-4. doi: 10.1016/j.archoralbio.2011.06.011. Epub 2011 Jul 8. Arch Oral Biol. 2012. PMID: 21741617
-
Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments.Appl Environ Microbiol. 2004 Feb;70(2):929-36. doi: 10.1128/AEM.70.2.929-936.2004. Appl Environ Microbiol. 2004. PMID: 14766573 Free PMC article.
-
Isolation and characterization of unsaturated fatty acid auxotrophs of Streptococcus pneumoniae and Streptococcus mutans.J Bacteriol. 2007 Nov;189(22):8139-44. doi: 10.1128/JB.01275-07. Epub 2007 Sep 7. J Bacteriol. 2007. PMID: 17827283 Free PMC article.
-
Role of unsaturated fatty acid biosynthesis in virulence of Streptococcus mutans.Infect Immun. 2007 Mar;75(3):1537-9. doi: 10.1128/IAI.01938-06. Epub 2007 Jan 12. Infect Immun. 2007. PMID: 17220314 Free PMC article.
-
The branched-chain amino acid aminotransferase encoded by ilvE is involved in acid tolerance in Streptococcus mutans.J Bacteriol. 2012 Apr;194(8):2010-9. doi: 10.1128/JB.06737-11. Epub 2012 Feb 10. J Bacteriol. 2012. PMID: 22328677 Free PMC article.
Cited by
-
Stress Physiology of Lactic Acid Bacteria.Microbiol Mol Biol Rev. 2016 Jul 27;80(3):837-90. doi: 10.1128/MMBR.00076-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27466284 Free PMC article. Review.
-
Streptococcus mutans protein synthesis during mixed-species biofilm development by high-throughput quantitative proteomics.PLoS One. 2012;7(9):e45795. doi: 10.1371/journal.pone.0045795. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049864 Free PMC article.
-
Streptococcus mutans: a new Gram-positive paradigm?Microbiology (Reading). 2013 Mar;159(Pt 3):436-445. doi: 10.1099/mic.0.066134-0. Epub 2013 Feb 7. Microbiology (Reading). 2013. PMID: 23393147 Free PMC article. Review.
-
Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes.Appl Environ Microbiol. 2007 Feb;73(3):997-1001. doi: 10.1128/AEM.00865-06. Epub 2006 Nov 17. Appl Environ Microbiol. 2007. PMID: 17114323 Free PMC article.
-
Current and prospective therapeutic strategies: tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm.Front Cell Infect Microbiol. 2023 May 11;13:1106231. doi: 10.3389/fcimb.2023.1106231. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37249973 Free PMC article. Review.
References
-
- Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. - PMC - PubMed
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

