Vibrio fischeri flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization
- PMID: 15205434
- PMCID: PMC421587
- DOI: 10.1128/JB.186.13.4315-4325.2004
Vibrio fischeri flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization
Abstract
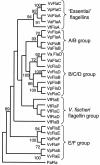
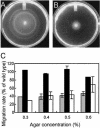
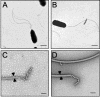
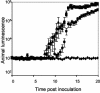
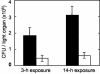
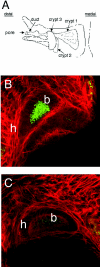
The motile bacterium Vibrio fischeri is the specific bacterial symbiont of the Hawaiian squid Euprymna scolopes. Because motility is essential for initiating colonization, we have begun to identify stage-specific motility requirements by creating flagellar mutants that have symbiotic defects. V. fischeri has six flagellin genes that are uniquely arranged in two chromosomal loci, flaABCDE and flaF. With the exception of the flaA product, the predicted gene products are more similar to each other than to flagellins of other Vibrio species. Immunoblot analysis indicated that only five of the six predicted proteins were present in purified flagella, suggesting that one protein, FlaF, is unique with respect to either its regulation or its function. We created mutations in two genes, flaA and flaC. Compared to a flaC mutant, which has wild-type flagellation, a strain having a mutation in the flaA gene has fewer flagella per cell and exhibits a 60% decrease in its rate of migration in soft agar. During induction of light organ symbiosis, colonization by the flaA mutant is impaired, and this mutant is severely outcompeted when it is presented to the animal as a mixed inoculum with the wild-type strain. Furthermore, flaA mutant cells are preferentially expelled from the animal, suggesting either that FlaA plays a role in adhesion or that normal motility is an advantage for retention within the host. Taken together, these results show that the flagellum of V. fischeri is a complex structure consisting of multiple flagellin subunits, including FlaA, which is essential both for normal flagellation and for motility, as well as for effective symbiotic colonization.
Figures
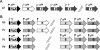

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

