Autologous tumor vaccines processed to express alpha-gal epitopes: a practical approach to immunotherapy in cancer
- PMID: 15205919
- PMCID: PMC11032768
- DOI: 10.1007/s00262-004-0524-x
Autologous tumor vaccines processed to express alpha-gal epitopes: a practical approach to immunotherapy in cancer
Abstract
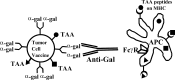
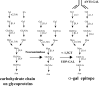
This review describes a method by which the human natural anti-Gal antibody can be exploited as an endogenous adjuvant for targeting autologous tumor vaccines to antigen-presenting cells (APCs). Tumor cells remaining in the patient after completion of surgery, radiation, and chemotherapy are the cause of tumor relapse. These residual tumor cells can not be detected by imaging, but their destruction may be feasible by active immunotherapy. Since specific tumor-associated antigens (TAAs) have not been identified for the majority of cancers, irradiated autologous tumor vaccines have been considered as an immunotherapy treatment that may elicit an immune response against the residual tumor cells expressing TAAs. However, tumor cells evolve in cancer patients in a stealthy way, i.e., they are not detected by APCs, even in the form of vaccine. Effective targeting of tumor vaccines for uptake by APCs is a prerequisite for eliciting an effective immune response which requires transport of the vaccine by APCs from the vaccination site to the draining lymph nodes. In the lymph nodes, the APCs transporting the vaccine process and present peptides, including the autologous TAA peptides for activation of the tumor-specific T cells. The required targeting of vaccines to APCs is feasible in humans by the use of anti-Gal. This antibody interacts specifically with the alpha-gal epitope (Galalpha1-3Galbeta1-4GlcNAc-R) and is the only known natural IgG antibody to be present in large amounts in all humans who are not severely immunocompromised. The alpha-gal epitope can be synthesized on any type of human tumor cell by the use of recombinant alpha1,3galactosyltransferase (alpha1,3GT). Solid tumors obtained from surgery are homogenized and their membranes subjected to alpha-gal epitope synthesis. Similarly, alpha-gal epitopes can be synthesized on intact tumor cells from hematological malignancies. Administration of irradiated autologous tumor vaccines processed to express alpha-gal epitopes results in in situ opsonization of the vaccinating cells or cell membranes due to anti-Gal binding to these epitopes. The bound antibody serves to target the autologous tumor vaccine to APCs because the Fc portion of the antibody interacts with Fcgamma receptors on APCs. Since patients receive their own TAAs, the vaccine is customized for autologous TAAs in the individual patient. The repeated vaccination with such autologous tumor vaccines provides the immune system of each patient with an additional opportunity to be effectively activated by the autologous TAAs. In some of the immunized patients this activation may be potent enough to induce an immune-mediated eradication of the residual tumor cells expressing these TAAs.
Figures
Similar articles
-
Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for alpha1,3galactosyltransferase.Cancer Res. 1999 Jul 15;59(14):3417-23. Cancer Res. 1999. PMID: 10416604
-
The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy.Immunol Cell Biol. 2005 Dec;83(6):674-86. doi: 10.1111/j.1440-1711.2005.01366.x. Immunol Cell Biol. 2005. PMID: 16266320 Review.
-
Anti-Gal-mediated targeting of human B lymphoma cells to antigen-presenting cells: a potential method for immunotherapy using autologous tumor cells.Haematologica. 2005 May;90(5):625-34. Haematologica. 2005. PMID: 15921377
-
Cancer immunotherapy for pancreatic cancer utilizing α-gal epitope/natural anti-Gal antibody reaction.World J Gastroenterol. 2015 Oct 28;21(40):11396-410. doi: 10.3748/wjg.v21.i40.11396. World J Gastroenterol. 2015. PMID: 26523105 Free PMC article. Review.
-
Preparation of autologous leukemia and lymphoma vaccines expressing alpha-gal epitopes.J Hematother Stem Cell Res. 2001 Aug;10(4):501-11. doi: 10.1089/15258160152509118. J Hematother Stem Cell Res. 2001. PMID: 11522233 Review.
Cited by
-
Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity.Cancer Immunol Immunother. 2009 Oct;58(10):1545-56. doi: 10.1007/s00262-009-0662-2. Epub 2009 Jan 28. Cancer Immunol Immunother. 2009. PMID: 19184002 Free PMC article.
-
Increasing Efficacy of Enveloped Whole-Virus Vaccines by In situ Immune-Complexing with the Natural Anti-Gal Antibody.Med Res Arch. 2021 Jul;9(7):2481. doi: 10.18103/mra.v9i7.2481. Epub 2021 Jul 10. Med Res Arch. 2021. PMID: 34853815 Free PMC article.
-
Immunotherapy with SLPI over-expressing mammary tumor cells decreases tumor growth.Cancer Immunol Immunother. 2011 Jun;60(6):895-900. doi: 10.1007/s00262-011-1018-2. Epub 2011 Apr 26. Cancer Immunol Immunother. 2011. PMID: 21519828 Free PMC article.
-
The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance.Biochim Biophys Acta. 2008 Feb;1780(2):75-88. doi: 10.1016/j.bbagen.2007.11.003. Epub 2007 Nov 22. Biochim Biophys Acta. 2008. PMID: 18047841 Free PMC article. Review.
-
Combination immunotherapy for high-risk resected and metastatic melanoma patients.Ochsner J. 2014 Summer;14(2):164-74. Ochsner J. 2014. PMID: 24940124 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

