Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity
- PMID: 15207503
- PMCID: PMC4034110
- DOI: 10.1016/j.it.2004.05.001
Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity
Abstract
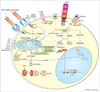
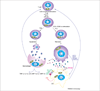
T-cell activation, proliferation and selection of the cell death pathway depend on the production of reactive oxygen intermediates (ROIs) and ATP synthesis, which are tightly regulated by the mitochondrial transmembrane potential (ΔΨm). Mitochondrial hyperpolarization (MHP) and ATP depletion represent early and reversible steps in T-cell activation and apoptosis. By contrast, T cells of patients with systemic lupus erythematosus (SLE) exhibit persistent MHP, cytoplasmic alkalinization, increased ROI production and depleted ATP, which mediate enhanced spontaneous and diminished activation-induced apoptosis and sensitize lupus T cells to necrosis. Necrotic, but not apoptotic, cell lysates activate dendritic cells and might account for increased interferon a production and inflammation in lupus patients. MHP is proposed as a key mechanism of SLE pathogenesis and is therefore a target for pharmacological intervention.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

