Plasticity of DNA replication initiation in Epstein-Barr virus episomes
- PMID: 15208711
- PMCID: PMC423133
- DOI: 10.1371/journal.pbio.0020152
Plasticity of DNA replication initiation in Epstein-Barr virus episomes
Abstract
In mammalian cells, the activity of the sites of initiation of DNA replication appears to be influenced epigenetically, but this regulation is not fully understood. Most studies of DNA replication have focused on the activity of individual initiation sites, making it difficult to evaluate the impact of changes in initiation activity on the replication of entire genomic loci. Here, we used single molecule analysis of replicated DNA (SMARD) to study the latent duplication of Epstein-Barr virus (EBV) episomes in human cell lines. We found that initiation sites are present throughout the EBV genome and that their utilization is not conserved in different EBV strains. In addition, SMARD shows that modifications in the utilization of multiple initiation sites occur across large genomic regions (tens of kilobases in size). These observations indicate that individual initiation sites play a limited role in determining the replication dynamics of the EBV genome. Long-range mechanisms and the genomic context appear to play much more important roles, affecting the frequency of utilization and the order of activation of multiple initiation sites. Finally, these results confirm that initiation sites are extremely redundant elements of the EBV genome. We propose that these conclusions also apply to mammalian chromosomes.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures
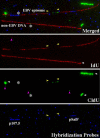

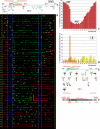
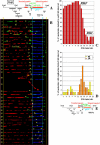

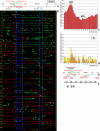
Similar articles
-
Visualization of DNA replication on individual Epstein-Barr virus episomes.Science. 2001 Dec 14;294(5550):2361-4. doi: 10.1126/science.1064603. Science. 2001. PMID: 11743204
-
Highly Efficient CRISPR/Cas9-Mediated Cloning and Functional Characterization of Gastric Cancer-Derived Epstein-Barr Virus Strains.J Virol. 2016 Apr 14;90(9):4383-93. doi: 10.1128/JVI.00060-16. Print 2016 May. J Virol. 2016. PMID: 26889033 Free PMC article.
-
Latent and lytic Epstein-Barr virus replication strategies.Rev Med Virol. 2005 Jan-Feb;15(1):3-15. doi: 10.1002/rmv.441. Rev Med Virol. 2005. PMID: 15386591 Review.
-
Chromatin Structure of Epstein-Barr Virus Latent Episomes.Curr Top Microbiol Immunol. 2015;390(Pt 1):71-102. doi: 10.1007/978-3-319-22822-8_5. Curr Top Microbiol Immunol. 2015. PMID: 26424644 Review.
-
De novo DNA methylation at nonrandom founder sites 5' from an unmethylated minimal origin of DNA replication in latent Epstein-Barr virus genomes.Biol Chem. 2000 Feb;381(2):95-105. doi: 10.1515/BC.2000.014. Biol Chem. 2000. PMID: 10746740
Cited by
-
LINEs of evidence: noncanonical DNA replication as an epigenetic determinant.Biol Direct. 2013 Sep 13;8:22. doi: 10.1186/1745-6150-8-22. Biol Direct. 2013. PMID: 24034780 Free PMC article. Review.
-
BLM helicase facilitates telomere replication during leading strand synthesis of telomeres.J Cell Biol. 2015 Jul 20;210(2):191-208. doi: 10.1083/jcb.201410061. J Cell Biol. 2015. PMID: 26195664 Free PMC article.
-
Timeless-dependent DNA replication-coupled recombination promotes Kaposi's Sarcoma-associated herpesvirus episome maintenance and terminal repeat stability.J Virol. 2013 Apr;87(7):3699-709. doi: 10.1128/JVI.02211-12. Epub 2013 Jan 16. J Virol. 2013. PMID: 23325691 Free PMC article.
-
DNA replication origins fire stochastically in fission yeast.Mol Biol Cell. 2006 Jan;17(1):308-16. doi: 10.1091/mbc.e05-07-0657. Epub 2005 Oct 26. Mol Biol Cell. 2006. PMID: 16251353 Free PMC article.
-
Telomeres and viruses: common themes of genome maintenance.Front Oncol. 2012 Dec 31;2:201. doi: 10.3389/fonc.2012.00201. eCollection 2012. Front Oncol. 2012. PMID: 23293769 Free PMC article.
References
-
- Anglana M, Apiou F, Bensimon A, Debatisse M. Dynamics of DNA replication in mammalian somatic cells: Nucleotide pool modulates origin choice and interorigin spacing. Cell. 2003;114:385–394. - PubMed
-
- Aston C, Hiort C, Schwartz DC. Optical mapping: An approach for fine mapping. Method Enzymol. 1999;303:55–73. - PubMed
-
- Babcock GJ, Hochberg D, Thorley-Lawson DA. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. - PubMed
-
- Bensimon A, Simon A, Chiffaudel A, Croquette V, Heslot F, et al. Alignment and sensitive detection of DNA by a moving interface. Science. 1994;265:2096–2098. - PubMed
-
- Berezney R, Dubey DD, Huberman JA. Heterogeneity of eukaryotic replicons, replicon clusters, and replication foci. Chromosoma. 2000;108:471–484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

