A specific interface between integrin transmembrane helices and affinity for ligand
- PMID: 15208712
- PMCID: PMC423134
- DOI: 10.1371/journal.pbio.0020153
A specific interface between integrin transmembrane helices and affinity for ligand
Abstract
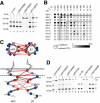
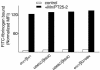
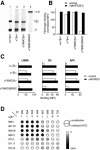
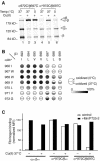
Conformational communication across the plasma membrane between the extracellular and intracellular domains of integrins is beginning to be defined by structural work on both domains. However, the role of the alpha and beta subunit transmembrane domains and the nature of signal transmission through these domains have been elusive. Disulfide bond scanning of the exofacial portions of the integrin alpha(IIbeta) and beta(3) transmembrane domains reveals a specific heterodimerization interface in the resting receptor. This interface is lost rather than rearranged upon activation of the receptor by cytoplasmic mutations of the alpha subunit that mimic physiologic inside-out activation, demonstrating a link between activation of the extracellular domain and lateral separation of transmembrane helices. Introduction of disulfide bridges to prevent or reverse separation abolishes the activating effect of cytoplasmic mutations, confirming transmembrane domain separation but not hinging or piston-like motions as the mechanism of transmembrane signaling by integrins.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures


Similar articles
-
Dissociation of the α-subunit Calf-2 domain and the β-subunit I-EGF4 domain in integrin activation and signaling.Biochemistry. 2010 Nov 30;49(47):10158-65. doi: 10.1021/bi101462h. Epub 2010 Nov 3. Biochemistry. 2010. PMID: 21038860
-
"Inside-out" signal transduction inhibited by isolated integrin cytoplasmic domains.J Biol Chem. 1994 Jul 15;269(28):18307-10. J Biol Chem. 1994. PMID: 8034576
-
Tests of integrin transmembrane domain homo-oligomerization during integrin ligand binding and signaling.J Biol Chem. 2011 Jan 21;286(3):1860-7. doi: 10.1074/jbc.M110.193797. Epub 2010 Nov 16. J Biol Chem. 2011. PMID: 21081497 Free PMC article.
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
-
Integrin regulation.Curr Opin Cell Biol. 2005 Oct;17(5):509-16. doi: 10.1016/j.ceb.2005.08.010. Curr Opin Cell Biol. 2005. PMID: 16099636 Review.
Cited by
-
Talin activates integrins by altering the topology of the β transmembrane domain.J Cell Biol. 2012 May 28;197(5):605-11. doi: 10.1083/jcb.201112141. J Cell Biol. 2012. PMID: 22641344 Free PMC article.
-
Consensus motif for integrin transmembrane helix association.Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):703-8. doi: 10.1073/pnas.0910873107. Epub 2009 Dec 18. Proc Natl Acad Sci U S A. 2010. PMID: 20080739 Free PMC article.
-
A push-pull mechanism for regulating integrin function.Proc Natl Acad Sci U S A. 2005 Feb 1;102(5):1424-9. doi: 10.1073/pnas.0409334102. Epub 2005 Jan 25. Proc Natl Acad Sci U S A. 2005. PMID: 15671157 Free PMC article.
-
A helix heterodimer in a lipid bilayer: prediction of the structure of an integrin transmembrane domain via multiscale simulations.Structure. 2011 Oct 12;19(10):1477-84. doi: 10.1016/j.str.2011.07.014. Structure. 2011. PMID: 22000516 Free PMC article.
-
Linking integrin conformation to function.J Cell Sci. 2009 Jan 15;122(Pt 2):165-70. doi: 10.1242/jcs.018556. J Cell Sci. 2009. PMID: 19118208 Free PMC article. Review.
References
-
- Armulik A, Nilsson I, von Heijne G, Johansson S. Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J Biol Chem. 1999;274:37030–37034. - PubMed
-
- Beglova N, Blacklow SC, Takagi J, Springer TA. Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat Struct Biol. 2002;9:282–287. - PubMed
-
- Buensuceso C, De Virgilio M, Shattil SJ. Detection of integrin αIIbβ3 clustering in living cells. J Biol Chem. 2003;278:15217–15224. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

