Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor
- PMID: 15209561
- PMCID: PMC1133867
- DOI: 10.1042/BJ20040762
Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor
Abstract
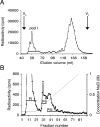
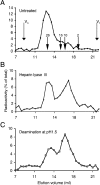
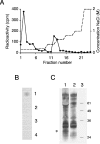
HS (heparan sulphate) has hitherto not been found on human red blood cells (RBCs, erythrocytes). However, malarial-parasite (Plasmodium falciparum)-infected RBCs adhere to uninfected RBCs via HS-like receptors. In the present paper we demonstrate that human RBCs carry epitopes for an anti-HS antibody. Glycans isolated from RBC membranes reacted to HS-specific degradations and adhered to an HS-binding malaria antigen. Additionally, an HS core protein was identified. This suggests that HS is present on human RBCs.
Figures

References
-
- Drzeniek Z., Stöcker G., Siebertz B., Just U., Schroeder T., Ostertag W., Haubeck H.-D. Heparan sulfate proteoglycan expression is induced during early erythroid differentiation of multipotent hematopoietic stem cells. Blood. 1999;93:2884–2897. - PubMed
-
- Miller L. H., Good M. F., Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. - PubMed
-
- Baruch D. I., Pasloske B. L., Singh H. B., Bi X., Ma X. C., Feldman M., Taraschi T. F., Howard R. J. Cloning the P. falciparum gene endcoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

