Amantadine therapy for chronic hepatitis C
- PMID: 15209605
- PMCID: PMC1492374
- DOI: 10.1111/j.1525-1497.2004.30057.x
Amantadine therapy for chronic hepatitis C
Abstract
Objective: Although treatment of hepatitis C has improved, up to 50% do not respond to standard therapy with interferon regimes or cannot tolerate the treatment due to side effects. The purpose of the present investigation was to evaluate the safety and effectiveness of the antiviral drug amantadine for the treatment of hepatitis C in those who had either previously failed interferon therapy or were not candidates for interferon.
Design: A prospective double-blind randomized placebo-controlled trial.
Setting: Outpatient research clinic of a teaching hospital.
Patients/participants: One hundred fifty-two patients with confirmed hepatitis C with abnormal liver enzymes, detectable hepatitis C RNA in the blood, and abnormal liver histology by biopsy were randomized to receive treatment or placebo.
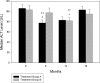
Measurements and main results: Patients received either amantadine 100 mg twice daily by mouth or placebo for 6 months. After 6 months, placebo-treated patients were crossed over and treated with amantadine for 6 months and amantadine-treated subjects received 6 additional months of therapy. Amantadine therapy resulted in a significant decline in serum alanine aminotransferase compared to placebo (P =.03). Nine percent cleared the virus at the end of therapy and 6.8% had a sustained virologic response 6 months after discontinuation of amantadine, but this was not statistically significant. Side effects were minimal, and the social quality of life survey improved with 12 months of amantadine (P =.02).
Conclusions: Oral amantadine may provide a safe alternative treatment for those patients who are intolerant or unresponsive to interferon.
Figures
Similar articles
-
Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C.Hepatology. 2000 Sep;32(3):630-4. doi: 10.1053/jhep.2000.16235. Hepatology. 2000. PMID: 10960460 Clinical Trial.
-
A randomized trial of amantadine and interferon versus interferon alone as initial treatment for chronic hepatitis C.Hepatology. 2001 Apr;33(4):989-93. doi: 10.1053/jhep.2001.23537. Hepatology. 2001. PMID: 11283865 Clinical Trial.
-
Combination of interferon alfa-2a and amantadine does not improve the efficacy of interferon therapy in patients with chronic hepatitis C.Hepatogastroenterology. 2003 Sep-Oct;50(53):1575-8. Hepatogastroenterology. 2003. PMID: 14571789 Clinical Trial.
-
[Evaluation of response to treatment in chronic hepatitis C].Gastroenterol Clin Biol. 2002 Apr;26 Spec No 2:B23-31. Gastroenterol Clin Biol. 2002. PMID: 12180294 Review. French. No abstract available.
-
Amantadine in treatment of chronic hepatitis C virus infection?J Viral Hepat. 2005 Sep;12(5):445-55. doi: 10.1111/j.1365-2893.2005.00622.x. J Viral Hepat. 2005. PMID: 16108758 Free PMC article. Review.
Cited by
-
Aminoadamantanes for chronic hepatitis C.Cochrane Database Syst Rev. 2014 May 3;2014(5):CD010125. doi: 10.1002/14651858.CD010125.pub2. Cochrane Database Syst Rev. 2014. PMID: 24793264 Free PMC article.
-
Long-term treatment with the combination of amantadine and ribavirin in hepatitis C nonresponders. A case series.Dig Dis Sci. 2007 Dec;52(12):3418-22. doi: 10.1007/s10620-007-9762-z. Epub 2007 Mar 31. Dig Dis Sci. 2007. PMID: 17401686
-
Aminoadamantanes versus other antiviral drugs for chronic hepatitis C.Cochrane Database Syst Rev. 2014 Jun 17;2014(6):CD011132. doi: 10.1002/14651858.CD011132.pub2. Cochrane Database Syst Rev. 2014. PMID: 24937404 Free PMC article.
References
-
- Laurence SP. Advances in the treatment of hepatitis C. Adv Intern Med. 2000;45:65–105. - PubMed
-
- Glue P, Rouzier-Pamis R, Raffanel C, et al. A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. Hepatology. 2000;32:647–53. - PubMed
-
- DiBisceglie AM, Hoofnagle JH. Optimal therapy of hepatitis C. Hepatology. 2002;36:S121–S127. - PubMed
-
- Trepo C. Genotype and viral load as prognostic indicators in the treatment of hepatitis C. J Viral Hepat. 2000;7:250–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
