Irbesartan inhibits human T-lymphocyte activation through downregulation of activator protein-1
- PMID: 15210574
- PMCID: PMC1575109
- DOI: 10.1038/sj.bjp.0705785
Irbesartan inhibits human T-lymphocyte activation through downregulation of activator protein-1
Abstract
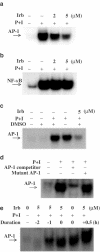
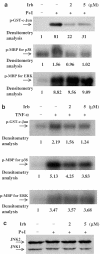
1 Irbesartan is a promising antihypertensive drug with beneficial effects on atherosclerotic processes. In the progression of atherosclerosis, human T-lymphocytes play an important role, but it is not yet known how irbesartan modulates human T-lymphocytes activation. To gain insight into the mechanisms by which irbesartan acts, we investigated its effects on human T-lymphocytes. 2 Primary human T-lymphocytes were isolated from whole blood. Cytokines were determined by ELISA. Activator protein-1 (AP-1) and related protein activities were determined by electrophoretic mobility shift assays, kinase assays, Western blotting and transfection assays. 3 Irbesartan inhibited the production of both tumor necrosis factor-alpha and interferon-gamma by activated T-cells, especially at therapeutic concentrations. Further investigation at the molecular level indicated that the inhibition of activated human T-lymphocytes specifically correlated with the downregulation of AP-1 DNA-binding activity. In the Jurkat T-cell line, irbesartan also inhibited AP-1 transcriptional activity. Finally, we revealed that irbesartan is unique in its ability to inhibit the activation of both c-Jun NH2-terminal protein kinase and p38 MAPK. 4 Our studies show that irbesartan may modulate inflammation-based atherosclerotic diseases through a cell-mediated mechanism involving suppression of human T-lymphocytes activation via downregulation of AP-1 activity.
Figures




References
-
- AHN J.D., MORISHITA R., KANEDA Y., LEE S.J., KWON K.Y., CHOI S.Y., LEE K.U., PARK J.Y., MOON I.J., PARK J.G., YOSHIZUMI M., OUCHI Y., LEE I.K. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ. Res. 2002;90:1325–1332. - PubMed
-
- BARATH P., FISHBEIN M.C., CAO J., BERENSON J., HELFANT R.H., FORRESTER J.S. Detection and localization of tumor necrosis factor in human atheroma. Am. J. Cardiol. 1990;65:297–302. - PubMed
-
- BERK B.C., VEKSHTEIN V., GORDON H.M., TSUDA T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989;13:305–314. - PubMed
-
- BRASIER A.R., RECINOS A., III, ELEDRISI M.S. Vascular inflammation and the renin–angiotensin system. Arterioscler. Thromb. Vasc. Biol. 2002;22:1257–1266. - PubMed
-
- BRUNNER H.R. The new angiotensin II receptor antagonist, irbesartan. Am. J. Hypertens. 1997;10:311S–317S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

