A randomised controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome
- PMID: 15210660
- PMCID: PMC1721707
- DOI: 10.1136/adc.2003.033555
A randomised controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome
Abstract
Background: The incidence of neonatal abstinence syndrome (NAS) has increased 10-fold over the last decade in Glasgow. In the Princess Royal Maternity Hospital, it now accounts for 17% of special care baby unit (SCBU) admissions.
Objective: To compare opiate replacement therapy (morphine sulphate) with the present standard treatment (phenobarbitone) for management of NAS. The primary study end point was duration of pharmaceutical treatment. Secondary end points were the requirement for additional drugs and the requirement for SCBU admission.
Design: Double blind, randomised controlled clinical trial.
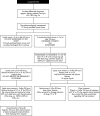
Methods: Differential diagnoses were excluded, and two consecutive Lipsitz scores > 4 defined NAS requiring treatment. Infants were randomised to receive morphine sulphate or phenobarbitone. Treatments were identical in appearance, odour, and volume. Increments, decrements, and discontinuation of treatments were protocol driven.
Results: Seventy five infants participated. All mothers received opiate replacement therapy (methadone) during pregnancy and most used other drugs (n = 62, 83%). No significant difference in maternal drug use patterns was observed between treatment groups. Median treatment duration was four days shorter with opiate replacement (8 v 12 days, Mann-Whitney U test, p = 0.02). Phenobarbitone treated infants tended to require second line treatment (47% v 35%, chi(2) test, p = 0.11) and SCBU admission (62% v 30%, chi(2) test, p = 0.04) more often.
Conclusions: Opiate replacement therapy appears to be superior for management of symptomatic NAS when maternal opiate use is prevalent. The shorter treatment duration and lower requirement for higher intensity nursing may have significant cost implications. Tailoring NAS treatment to local maternal drug use may result in similar benefits.
Figures
Similar articles
-
Management of neonatal abstinence syndrome in neonates born to opioid maintained women.Drug Alcohol Depend. 2007 Mar 16;87(2-3):131-8. doi: 10.1016/j.drugalcdep.2006.08.024. Epub 2006 Sep 26. Drug Alcohol Depend. 2007. PMID: 17000060 Clinical Trial.
-
[Opiate addiction in gravidity - consequences for the newborn. Results of an interdisciplinary treatment concept].Z Geburtshilfe Neonatol. 2001 Nov-Dec;205(6):224-30. doi: 10.1055/s-2001-19054. Z Geburtshilfe Neonatol. 2001. PMID: 11745008 German.
-
[Drug withdrawal in newborns - clinical data of 49 infants with intrauterine drug exposure: what should be done?].Klin Padiatr. 2008 Sep-Oct;220(5):308-15. doi: 10.1055/s-2007-992800. Epub 2008 Feb 7. Klin Padiatr. 2008. PMID: 18260044 German.
-
Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates.Addiction. 2008 Sep;103(9):1429-40. doi: 10.1111/j.1360-0443.2008.02283.x. Addiction. 2008. PMID: 18783498 Review.
-
Update on the pharmacologic management of neonatal abstinence syndrome.J Perinatol. 2011 Nov;31(11):692-701. doi: 10.1038/jp.2011.116. Epub 2011 Aug 25. J Perinatol. 2011. PMID: 21869765 Review.
Cited by
-
Neonatal abstinence syndrome: essentials for the practitioner.J Pediatr Pharmacol Ther. 2014 Jul;19(3):147-55. doi: 10.5863/1551-6776-19.3.147. J Pediatr Pharmacol Ther. 2014. PMID: 25309144 Free PMC article. Review.
-
Phenobarbital versus morphine in the management of neonatal abstinence syndrome, a randomized control trial.BMC Pediatr. 2015 May 15;15:57. doi: 10.1186/s12887-015-0377-9. BMC Pediatr. 2015. PMID: 25976238 Free PMC article. Clinical Trial.
-
Pragmatic, randomized, blinded trial to shorten pharmacologic treatment of newborns with neonatal opioid withdrawal syndrome (NOWS).Trials. 2023 Jul 21;24(1):466. doi: 10.1186/s13063-023-07378-x. Trials. 2023. PMID: 37480087 Free PMC article. Clinical Trial.
-
Neonatal Abstinence Syndrome and Corresponding Pharmacotherapy Approaches from 2 University-affiliated Neonatal Intensive Care Units in Puerto Rico (2018-2020).P R Health Sci J. 2024 Mar;43(1):25-31. P R Health Sci J. 2024. PMID: 38512758 Free PMC article.
-
Pharmacological Treatments for Neonatal Abstinence Syndrome: A Systematic Review and Network Meta-analysis.JAMA Pediatr. 2019 Mar 1;173(3):234-243. doi: 10.1001/jamapediatrics.2018.5044. JAMA Pediatr. 2019. PMID: 30667476 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical