SUMOylation regulates nucleo-cytoplasmic shuttling of Elk-1
- PMID: 15210726
- PMCID: PMC2172397
- DOI: 10.1083/jcb.200310136
SUMOylation regulates nucleo-cytoplasmic shuttling of Elk-1
Abstract
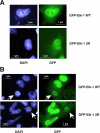
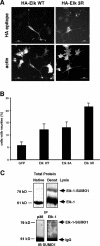
The transcription factor Elk-1 is a nuclear target of mitogen-activated protein kinases and regulates immediate early gene activation by extracellular signals. We show that Elk-1 is also conjugated to SUMO on either lysines 230, 249, or 254. Mutation of all three sites is necessary to fully block SUMOylation in vitro and in vivo. This Elk-1 mutant, Elk-1(3R), shuttles more rapidly to nuclei of Balb/C cells fused to transfected HeLa cells. Coexpression of SUMO-1 or -2 strongly reduces shuttling by Elk-1 without affecting that of Elk-1(3R), indicating that SUMOylation regulates nuclear retention of Elk-1. Accordingly, overexpression of Elk-1(3R) in PC12 cells, where cytoplasmic relocalization of Elk-1 has been linked to differentiation, enhances neurite extension relative to Elk-1. The effect of Elk-1, but not of the 3R mutant, was blocked upon cotransfection with SUMO-1 or -2 and enhanced by coexpression with mutant Ubc-9. Thus, SUMO conjugation is a novel regulator of Elk-1 function through the control of its nuclear-cytoplasmic shuttling.
Figures



Similar articles
-
CSF-1 induces fos gene transcription and activates the transcription factor Elk-1 in mature osteoclasts.Calcif Tissue Int. 2005 May;76(5):371-8. doi: 10.1007/s00223-004-0099-8. Epub 2005 Apr 11. Calcif Tissue Int. 2005. PMID: 15812575
-
SUMO regulates the cytoplasmonuclear transport of its target protein Daxx.J Cell Biochem. 2006 Jul 1;98(4):895-911. doi: 10.1002/jcb.20703. J Cell Biochem. 2006. PMID: 16475184
-
Down-regulation of c-Fos/c-Jun AP-1 dimer activity by sumoylation.Mol Cell Biol. 2005 Aug;25(16):6964-79. doi: 10.1128/MCB.25.16.6964-6979.2005. Mol Cell Biol. 2005. PMID: 16055710 Free PMC article.
-
SUMO and transcriptional repression: dynamic interactions between the MAP kinase and SUMO pathways.Cell Cycle. 2003 Nov-Dec;2(6):528-30. doi: 10.4161/cc.2.6.597. Cell Cycle. 2003. PMID: 14504467 Review.
-
Nuclear and unclear functions of SUMO.Nat Rev Mol Cell Biol. 2003 Sep;4(9):690-9. doi: 10.1038/nrm1200. Nat Rev Mol Cell Biol. 2003. PMID: 14506472 Review.
Cited by
-
Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1.Mol Cell Biol. 2005 Nov;25(22):9874-85. doi: 10.1128/MCB.25.22.9874-9885.2005. Mol Cell Biol. 2005. PMID: 16260603 Free PMC article.
-
Regulation of transforming growth factor-β signalling by SUMOylation and its role in fibrosis.Open Biol. 2021 Nov;11(11):210043. doi: 10.1098/rsob.210043. Epub 2021 Nov 10. Open Biol. 2021. PMID: 34753319 Free PMC article.
-
Interaction of porcine reproductive and respiratory syndrome virus proteins with SUMO-conjugating enzyme reveals the SUMOylation of nucleocapsid protein.PLoS One. 2017 Dec 13;12(12):e0189191. doi: 10.1371/journal.pone.0189191. eCollection 2017. PLoS One. 2017. PMID: 29236778 Free PMC article.
-
Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana.Protein Sci. 2008 Oct;17(10):1771-80. doi: 10.1110/ps.035121.108. Epub 2008 Aug 20. Protein Sci. 2008. PMID: 18715992 Free PMC article.
-
A guide to ERK dynamics, part 1: mechanisms and models.Biochem J. 2023 Dec 13;480(23):1887-1907. doi: 10.1042/BCJ20230276. Biochem J. 2023. PMID: 38038974 Free PMC article.
References
-
- Estrach, S., S. Schmidt, S. Diriong, A. Penna, A. Blangy, P. Fort, and A. Debant. 2002. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 12:307–312. - PubMed
-
- Janknecht, R., R. Zinck, W.H. Ernst, and A. Nordheim. 1994. Functional dissection of the transcription factor Elk-1. Oncogene. 9:1273–1278. - PubMed
-
- Kim, K.I., S.H. Baek, and C.H. Chung. 2002. Versatile protein tag, SUMO: its enzymology and biological function. J. Cell. Physiol. 191:257–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

